|
|
Post by peppy on Sept 11, 2018 11:15:41 GMT -5
Thank you Harry. www.screencast.com/t/t9SQOnIE8My eyeballs went straight to COPD. www.accessdata.fda.gov/drugsatfda_docs/label/2004/20549slr016,20548slr020,20121slr030_flonase_lbl.pdf Salmeterol all product name for fluticasone? see the "sone"at the end of fluticasone? bethamethasone? a steriod. same old crap, different day. so these stop the immune system, reduce the fluid? They do not do anything about the clog, and the decreased compliance. hmmmm. Thank you again Harry. I am just looking. The model below showing it is the muscle that is not longer compliant. I thought it was the alveoli it's self. There it is, the loss of alveolar elasticity. multiple components. added: still it is the loss of the elasticity, I am not sure why medicine does not go that way. Perhaps someone can tell me. *Surfactant, a different medication. www.accessdata.fda.gov/drugsatfda_docs/label/2012/021746s000lbl.pdf12.1 Mechanism of Action Endogenous pulmonary surfactant l owers surface tension at the air-liquid interface of the alveolar surfaces during respiration and stabilizes the alveoli against collapse at resting transpulmonary pressures. A deficiency of pulmonary surfactant in premature infants results in RDS. SURFAXIN compensates for the deficiency of surfactant and restores surface activity to the lungs of these infants. In vitro Lucinactant lowers minimum surface tension to ≤ 6 dynes per cm, as assessed by the pulsating bubble surfactometer. 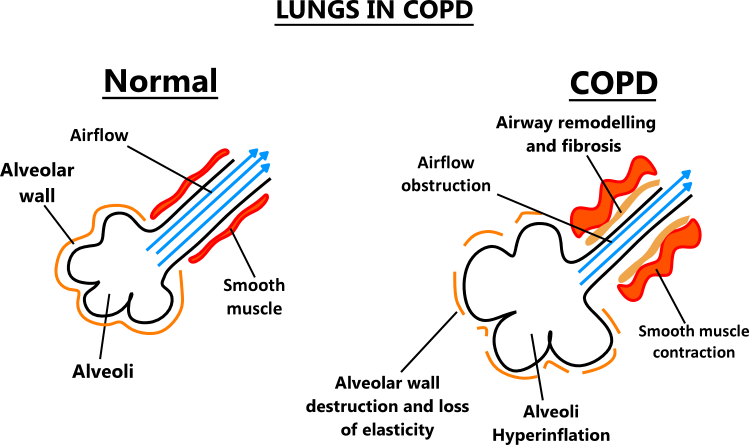  |
|
|
|
Post by peppy on Sept 11, 2018 12:16:57 GMT -5
|
|
|
|
Post by Clement on Sept 11, 2018 13:57:29 GMT -5
Thanks, Harry, I see Advair now-- the one on the bottom -- fluticasone/salmeterol. Facing a patent cliff! |
|
|
|
Post by mango on Sept 11, 2018 16:49:07 GMT -5
Thank you Harry. www.screencast.com/t/t9SQOnIE8My eyeballs went straight to COPD. www.accessdata.fda.gov/drugsatfda_docs/label/2004/20549slr016,20548slr020,20121slr030_flonase_lbl.pdf Salmeterol all product name for fluticasone? see the "sone"at the end of fluticasone? bethamethasone? a steriod. same old crap, different day. so these stop the immune system, reduce the fluid? They do not do anything about the clog, and the decreased compliance. hmmmm. Thank you again Harry. I am just looking. The model below showing it is the muscle that is not longer compliant. I thought it was the alveoli it's self. There it is, the loss of alveolar elasticity. multiple components. added: still it is the loss of the elasticity, I am not sure why medicine does not go that way. Perhaps someone can tell me. *Surfactant, a different medication. www.accessdata.fda.gov/drugsatfda_docs/label/2012/021746s000lbl.pdf12.1 Mechanism of Action Endogenous pulmonary surfactant l owers surface tension at the air-liquid interface of the alveolar surfaces during respiration and stabilizes the alveoli against collapse at resting transpulmonary pressures. A deficiency of pulmonary surfactant in premature infants results in RDS. SURFAXIN compensates for the deficiency of surfactant and restores surface activity to the lungs of these infants. In vitro Lucinactant lowers minimum surface tension to ≤ 6 dynes per cm, as assessed by the pulsating bubble surfactometer.   The smooth muscle. The airways are constantly ungoing remodeling and repairing but bc the lungs are under continuous inflammation etc things don't really work out for the person's benefit. If we could develop a drug that could activate/interact with the body's cells that play a key role in lung tissue homeostasis, like c-kit, while also commanding the immune cells, maybe through the CB2 receptor, maybe some meaningful remodeling and repair in chronically diseased lungs could happen? COPD = high lung compliance (the elasticiity). There is chronic poor gas exchange, connective tissue is chronically damaged, airways narrowed and dilated, atelectasis etc C-kit is vital for lung tissue homeostasis. Interestingly, it is a receptor tyrosine kinase protein and is capable of self-renewal, along with some other cool stuff. It's also interesting that the aveolar type 2 cells have cannabinoid receptors. These cells produce pulmonary surfactant. It is no suprise that asthmatics have relief with Cannabis. Bronchodilator. Relaxes the smooth muscles. There is a famous clinical study... I wonder which terpenoids have bronchodilator effect...maybe RLS knows There is also a lot of information with CBD and graft versus host. This makes sense too because CBD is an endocannabinoid modulator and CB2 modulates immune cell functions. Raphael Mechoulam is doing work in this area with Kalytera Therapeutics. Below is the highlights from the Phase 2a study Phase 2a Study Highlights • No patients developed acute GVHD while being treated with CBD • The risk of developing acute GVHD by day 100 was decreased • Among those that did develop GVHD after HCT, the time to onset was significantly longer (60 days in the CBD group versus 20 days in the group versus) • Patients treated with CBD had fewer skin and gastrointestinal issues compared to the control group • CBD treatment was safe and well tolerated I wonder if United has thought about working with RLS to develop a cannabinoid therapy to compliment their lung transplant program. Probably would be a lot better than pursuing Tacrolimus |
|
|
|
Post by peppy on Sept 12, 2018 7:07:58 GMT -5
put this under crazy talk. If there is anything to CBD and say arthritis. Immune réponse the white blood cell count. The immune cells. there they are.Neutrophils, lymphocytes, Monocytes, Eosinophils,basophils. Anybody see the humor if CBD works to slow down the toxins used by these cells to encompass?  (What are the components of the complete blood count (CBC)? The complete blood count, or CBC, lists a number of many important values. Typically, it includes the following: White blood cell count (WBC or leukocyte count) WBC differential count Red blood cell count (RBC or erythrocyte count) Hematocrit (Hct)) |
|
|
|
Post by liane on Sept 12, 2018 7:28:10 GMT -5
Folk - can you please take the off topic stuff to the appropriate forum. Posts in this thread should focus directly on Technosphere apps.
|
|
|
|
Post by anderson on Sept 12, 2018 9:10:24 GMT -5
and Pfizer’s rights under a license to manufacture insulin for pulmonary delivery.
Don't think they used any of it since they used the Amphastar insulin for the trials. Al said the pfizer insulin was in deep storage and monitored. See this thread for info on the insulin stockpile.
The Pfizer insulin was used from back in 2009, I don't think Amphastar got involved until around 2014 and the Sanofi deal, but I could well be wrong on that. In the intervening five years I understood that they used the Pfizer insulin. At the end of the day it's all Regular insulin so, FDA and swapping APIs aside, it should be a trivial to interchange them. The Pfizer insulin is in deep storage and monitored, that's what Mannkind were paid $3M for, but it is still owned by Pfizer. Still incorrect. The Pfizer insulin was never used as the API(well maybe engineering samples). Yes the $3 million was charged to R&D cost but does not mean it was used. The insulin used in the API was from N.V. Organon a subsidiary of Merck, and is the API that is FDA approved for Afrezza. So where does Amphastar come in. Well in April 2014 they bought that insulin manufacturing facility from N.V. Organon as noted here,
So the treprostinil will give them a pattern for boi similarity trials. And once they get the cash they should to be able to get another API approved. It is only prudent to have more than one source for the API. Also 8 years of storage cost and the agreement to be able to buy the rest of the Pfizer insulin at a low price should make the several tons of it they have sitting around affordable to do whatever testing they need to. But without a plant to produce more it is pointless to do the trials. Sanofi bought the Pfizer plant so MNKD needs the cash to build its own to make this pipe dream to reality.
|
|
|
|
Post by agedhippie on Sept 12, 2018 9:35:01 GMT -5
The Pfizer insulin was used from back in 2009, I don't think Amphastar got involved until around 2014 and the Sanofi deal, but I could well be wrong on that. In the intervening five years I understood that they used the Pfizer insulin. At the end of the day it's all Regular insulin so, FDA and swapping APIs aside, it should be a trivial to interchange them. The Pfizer insulin is in deep storage and monitored, that's what Mannkind were paid $3M for, but it is still owned by Pfizer. Still incorrect. The Pfizer insulin was never used as the API(well maybe engineering samples). Yes the $3 million was charged to R&D cost but does not mean it was used.
... I have never said that it was used as the API. Purely in practical terms it would never be a a viable option as an API since there was a finite supply. That is no issue for engineering though which is what was being done at the time. |
|
|
|
Post by michaelb on Sept 12, 2018 15:55:52 GMT -5
I think Palonosetron could be a good next application for MNKD as the sister compound Ondansetron has had increasing off label use for many kinds of nausea and vomiting conditions. For example my primary care doc recently offered it to me during a GI viral illness. I have used Ondansetron during a bout of prolonged vertigo and for motion sickness and it is very effective. The oral medication has a 45 minute time to effect and so an inhaled formulation and shorter action would be really nice. Also, constipation is a major side effect after use and I wonder if this might be less with a technosphere product as my guess is this side effect is driven by the serotonin receptors in the gut which might have less impact with an inhaled treatment. Both meds are serotonin 5-HT3 antagonists. Hope I am posting something already on the board.
|
|
|
|
Post by michaelb on Sept 12, 2018 15:56:29 GMT -5
oops I meant "not" already on the board
|
|
|
|
Post by patten1962 on Sept 13, 2018 4:23:00 GMT -5
Sept 4th, 4pm webcast by Mike C.
Durning 1st question by H.C.Wainwright, Mike said, UTHR has an "Investor" call at the end of Sept. This is not the earnings call. That is Oct 23rd.
I am hoping UTHR has something to say that Mike can talk about at the Cantor call on 10/2.
Did anyone else pick up on this?
Any thoughts?
And yes, went to UTHR website, did not see any call.
|
|
|
|
Post by sportsrancho on Sept 13, 2018 6:03:36 GMT -5
Mike had some other things to say but said he didn’t want to get ahead of them.
I can’t find any information on where or when their investor call is. I mistakenly thought he meant their next earnings call.
|
|
|
|
Post by mnholdem on Sept 13, 2018 7:01:13 GMT -5
UTC’s conference call could simply be about their buyout of PAH competitor SteadyMed. The M&A just closed the other day.
|
|
|
|
Post by mango on Sept 13, 2018 7:04:56 GMT -5
I wonder if United has thought about partnering with these two companies at the Alfred Mann Institute at the Technion. Accellta offers a range of revolutionary solutions for better affordability and quality of stem cell culturing. Accellta's technologies are the result of over 15 years of pioneering research and development at the Stem Cell Center in the Technion’s Rappaport Faculty of Medicine. Accellta provides custom-made solutions for mass production of high quality human embryonic and induced pluripotent stem cells, progenitors and differentiated cells for drug discovery, regenerative medicine and research. Culturing is conducted in 3D suspension systems under feeder-free, adherent-free and xeno-free conditions. The tailored solutions dramatically reduce production time and costs.www.accellta.comSealantis develops innovative products based on a proprietary platform of alga-mimetic tissue adhesives, for a variety of applications and clinical needs in surgical adhesion, leakage control, adhesion-prevention and drug delivery. The Sealantis adhesive technology mimics the underwater adherence mechanism of algae, providing it with a superior ability to stick to tissues and grafts even in a wet environment. The adhesives are bioresorbable (degrade in the body) and do not contain proteins (thus eliminate potential immunogenic and allergic risks).
www.sealantis.co.il |
|
|
|
Post by patten1962 on Sept 14, 2018 19:30:45 GMT -5
Sept 4th, 4pm webcast by Mike C. Durning 1st question by H.C.Wainwright, Mike said, UTHR has an "Investor" call at the end of Sept. This is not the earnings call. That is Oct 23rd. I am hoping UTHR has something to say that Mike can talk about at the Cantor call on 10/2. Did anyone else pick up on this? Any thoughts? And yes, went to UTHR website, did not see any call. I emailed UTHR about the investor call. Waiting fo an answer. |
|