|
|
Post by mnholdem on Jul 3, 2018 14:49:54 GMT -5
IMO how trial data gets presented to the medical community can be more important than the raw data itself. CMO David Kendall stated at the recent investors conference that the first publication of data presented at ADA2018 will happen within the next two weeks.
|
|
|
|
Post by agedhippie on Jul 3, 2018 17:29:34 GMT -5
Again, common sense, I will take my lead from the qualified chief medical officer who was a former head of the ADA and chief global scientist for one of the leading BP's in the space rather than someone that trolls the boards with ulterior motives and recommends via direct messaging to people to sell their shares  thank you. So which bit of Matt's assessment do you disagree with? - The evidence requirement is smack on the money. - The observation that STAT was too small to influence the standard of care was also correct (even Mannkind described it as a pilot). STAT was useful in that it showed that a second bigger trial (STAT-2?) was worth funding, but as a tool to change the Standard of Care it is never going to do it. And Chief Global Scientist is a nice title, if fictitious, and not one that Dr Kendall ever claimed or could have claimed given how Eli Lilly is structured and his role there. |
|
|
|
Post by sayhey24 on Jul 3, 2018 17:46:36 GMT -5
Aged - I have to agree with Joey and Dr. Kendall on this. I think Dr. Kendall said it was the easiest job he ever had. He has the Affinity-1 with 500+ participants which demonstrate A1c 8 to 6.8 with no additional hypoglycemia. He has the 171 which mirrors the non-compliant STAT patients. He has the compliant group which mimic the clinical results of the current PWDs MNKD has been tracking. Additionally he has a gold mine in other study info which has not yet been released.
How much more is required? The STAT is a pilot to provide insight into other trial results and Dr. Kendall has a ton. While I know you keep saying he does not, I am not sure why he would say this is easy? You could be right and he could be wrong and if so maybe you should be the CMO. But, I got to tell you, my money is on him.
He will need more for the T2s but he should at least get a mention of inhaled insulin. In 18 months he can close the deal on the T2s but he should be able to close the T1 deal in 6 months along with the T2 mention.
|
|
|
|
Post by sayhey24 on Jul 3, 2018 17:54:41 GMT -5
One more thing, he has some of the leading thought leaders on his advisory board. I think he has level 1, 2 and 3 covered.
|
|
|
|
Post by mango on Jul 3, 2018 18:47:21 GMT -5
No manufacturer can compel the ADA to do anything. STAT, while helpful, did not exactly provide earth shattering results as the conclusion was pretty much predetermined. Physicians look to "levels of evidence" when making treatment recommendations and if you are not familiar with the levels, in the United States these are: Level I: Evidence obtained from at least one properly designed randomized controlled trial. Level II-1: Evidence obtained from well-designed controlled trials without randomization. Level II-2: Evidence obtained from well-designed cohort or case-control analytic studies, preferably from more than one center or research group. Level II-3: Evidence obtained from multiple time series designs with or without the intervention. Dramatic results in uncontrolled trials might also be regarded as this type of evidence. Level III: Opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees Some countries have additional levels of evidence but the framework is similar. STAT was not a controlled trial since only the patients on the Afrezza arm were allowed to adjust their insulin dose following the meal in response to the CGM. That makes it a Level III study at best, and that is the least persuasive form of evidence. If MNKD wants to move the needle they need to sponsor a large randomized mullti-center controlled trial where all patients are given the opportunity to adjust their insulin dose after seeing the results from a glucose meter. If the results from that study demonstrate that time in range is statistically better with Afrezza than other RAI products, or if Afrezza has similar time in range but with fewer hypoglycemic events that require a visit to the ER, then they will have a compelling argument. The two big problems with STAT are these: 1. The cohort sizes were too small. In order to get the necessary statistical power, a proper study would require 300-500 patients over a longer period such as 90-180 days. 2. Allowing the Afrezza patients to adjust their dosing in response to the CGM results, not once but twice, stacked the deck against the comparator. Both arms must be allowed to adjust the dose after seeing the initial results for the trial to be credible. Some will say that patients on injectable insulin will avoid multiple needle sticks or that they will have too many severe hypo events, but that is precisely what the trial needs to show. If so many needle sticks is a major issue then patients on RAI will not be in range as long, and if the second bolus causes hypo events that should show up in the data as well. Opinions are not Level I evidence; data from a well-designed trial is evidence. Level I evidence, preferably with some parallel economic evidence showing that Afrezza is cost effective, is what it will take. The label for insulin Aspart: • Subcutaneous injection Novolog: —5-10 minutes before a meal Fiasp: —Inject at the start of a meal or within 20 minutes after starting a meal • The investigator that initiated the trial had the participants in the insulin Aspart arm to continue their usual diabetes care, which is seen above, per the label. It was not the investigator's job to do what you think should have been done, causing a butt load of severe hypos just to prove a point. The point has been proven without needless suffering—Afrezza is superior to insulin Aspart. • STAT was an investigator-initiated, prospective, randomized, multicenter, parallel, open-label, pilot clinical trial. • The participant size was sufficient and adequate. I am waiting for you to provide the appropriate literature which states otherwise. • Once a living Standards update recommendation has been received by the ADA home office and/or the PPC Chair: 1. A proposed language change will be drafted within the relevant section(s) of the most current Standards. 2. A narrative summary and justification (with supporting literature) and the proposed living Standards update will be distributed to the PPC for review. 3. An email vote will be taken from members of the PPC to determine: —Is the change sufficiently notable to warrant a living Standards update? —Is/are the proposed edit(s) acceptable as proposed, or are there any suggestions for revision? —A majority vote (>50% agreement) of the PPC will be required to ratify a proposed living Standards update. 4. If a proposed living Standards update is approved by a PPC majority, the PPC Chair will submit a final summary of the approved change to ADA for processing. 5. Updates will be posted online as annotations in Diabetes Care (full Standards) and, when appropriate, Clinical Diabetes (abridged Standards). 6. ADA will update the Standards slide deck, Standards app, professional education programs, and selected other materials to be consistent with living Standards updates. |
|
|
|
Post by agedhippie on Jul 3, 2018 19:14:53 GMT -5
The label for insulin Aspart: • Subcutaneous injection Novolog: —5-10 minutes before a meal ... • The investigator that initiated the trial had the participants in the insulin Aspart arm to continue their usual diabetes care, which is seen above, per the label. It was not the investigator's job to do what you think should have been done, causing a butt load of severe hypos just to prove a point. The point has been proven without needless suffering—Afrezza is superior to insulin Aspart. • STAT was an investigator-initiated, prospective, randomized, multicenter, parallel, open-label, pilot clinical trial. • The participant size was sufficient and adequate. I am waiting for you to provide the appropriate literature which states otherwise. You have the label for Novolog wrong, it says is " NovoLog should generally be given immediately (within 5-10 minutes) prior to the start of a meal." In other words dose at the start of the meal, and if you pre-bolus do not do so by more than 10 minutes. The Afrezza arm is flawed because the investigator intensified the Afrezza arm (the Afrezza label says, " Administer at the beginning of a meal.") by changing the dosing from the label, but did not do the same for the Novolog arm. Arguing that you left people to their own devices is fine, but you are then comparing an intensified regime with a regular regime so of course the intensified regime performed better. Actually STAT was not multi-centered, it was carried out at Barbara Davis Center, Aurora, Colorado. That's a single location. There were enough participants for a pilot study, there were not enough participants for a pivotal study. Changing the Standard of Care will take a pivotal study. |
|
|
|
Post by agedhippie on Jul 3, 2018 19:36:20 GMT -5
Aged - I have to agree with Joey and Dr. Kendall on this. I think Dr. Kendall said it was the easiest job he ever had. He has the Affinity-1 with 500+ participants which demonstrate A1c 8 to 6.8 with no additional hypoglycemia. He has the 171 which mirrors the non-compliant STAT patients. He has the compliant group which mimic the clinical results of the current PWDs MNKD has been tracking. Additionally he has a gold mine in other study info which has not yet been released. How much more is required? The STAT is a pilot to provide insight into other trial results and Dr. Kendall has a ton. While I know you keep saying he does not, I am not sure why he would say this is easy? You could be right and he could be wrong and if so maybe you should be the CMO. But, I got to tell you, my money is on him. He will need more for the T2s but he should at least get a mention of inhaled insulin. In 18 months he can close the deal on the T2s but he should be able to close the T1 deal in 6 months along with the T2 mention. You realize that AFFINITY-1 and 171 are different names for the same trial? Equally that A1c was reduced by Afrezza in that trial on average by 0.21 to 7.77 and not 6.8. Today compliant Afrezza users are those who take a single dose at meal time. If that is to be changed it will take trial data, STAT-2 would be a prime candidate for that vehicle. I do think Dr Kendall can get inhaled insulin into the Type 1 recommendations by year end. I am curious to see if the ADA add inhaled insulin, or generalize the recommendation by removing the reference to injections. |
|
|
|
Post by mnkdfann on Jul 3, 2018 20:02:28 GMT -5
IMO how trial data gets presented to the medical community can be more important than the raw data itself. CMO David Kendall stated at the recent investors conference that the first publication of data presented at ADA2018 will happen within the next two weeks. Let's hope it is in a good refereed journal. Not a throwaway journal. Something much better than a Hindawi or Bentham journal (Hindawi being where a previous article about Afrezza appeared.) |
|
|
|
Post by sportsrancho on Jul 3, 2018 20:03:37 GMT -5
No manufacturer can compel the ADA to do anything. STAT, while helpful, did not exactly provide earth shattering results as the conclusion was pretty much predetermined. Physicians look to "levels of evidence" when making treatment recommendations and if you are not familiar with the levels, in the United States these are: Level I: Evidence obtained from at least one properly designed randomized controlled trial. Level II-1: Evidence obtained from well-designed controlled trials without randomization. Level II-2: Evidence obtained from well-designed cohort or case-control analytic studies, preferably from more than one center or research group. Level II-3: Evidence obtained from multiple time series designs with or without the intervention. Dramatic results in uncontrolled trials might also be regarded as this type of evidence. Level III: Opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees Some countries have additional levels of evidence but the framework is similar. STAT was not a controlled trial since only the patients on the Afrezza arm were allowed to adjust their insulin dose following the meal in response to the CGM. That makes it a Level III study at best, and that is the least persuasive form of evidence. If MNKD wants to move the needle they need to sponsor a large randomized mullti-center controlled trial where all patients are given the opportunity to adjust their insulin dose after seeing the results from a glucose meter. If the results from that study demonstrate that time in range is statistically better with Afrezza than other RAI products, or if Afrezza has similar time in range but with fewer hypoglycemic events that require a visit to the ER, then they will have a compelling argument. The two big problems with STAT are these: 1. The cohort sizes were too small. In order to get the necessary statistical power, a proper study would require 300-500 patients over a longer period such as 90-180 days. 2. Allowing the Afrezza patients to adjust their dosing in response to the CGM results, not once but twice, stacked the deck against the comparator. Both arms must be allowed to adjust the dose after seeing the initial results for the trial to be credible. Some will say that patients on injectable insulin will avoid multiple needle sticks or that they will have too many severe hypo events, but that is precisely what the trial needs to show. If so many needle sticks is a major issue then patients on RAI will not be in range as long, and if the second bolus causes hypo events that should show up in the data as well. Opinions are not Level I evidence; data from a well-designed trial is evidence. Level I evidence, preferably with some parallel economic evidence showing that Afrezza is cost effective, is what it will take. Again, common sense, I will take my lead from the qualified chief medical officer who was a former head of the ADA and chief global scientist for one of the leading BP's in the space rather than someone that trolls the boards with ulterior motives and recommends via direct messaging to people to sell their shares  thank you. Boy, if I didn’t know better, ( ha ha ) I’d think there was big money bet on the stock in both directions... if this thread doesn’t prove that I don’t know what does. |
|
|
|
Post by sayhey24 on Jul 4, 2018 6:34:58 GMT -5
Aged - I have to agree with Joey and Dr. Kendall on this. I think Dr. Kendall said it was the easiest job he ever had. He has the Affinity-1 with 500+ participants which demonstrate A1c 8 to 6.8 with no additional hypoglycemia. He has the 171 which mirrors the non-compliant STAT patients. He has the compliant group which mimic the clinical results of the current PWDs MNKD has been tracking. Additionally he has a gold mine in other study info which has not yet been released. How much more is required? The STAT is a pilot to provide insight into other trial results and Dr. Kendall has a ton. While I know you keep saying he does not, I am not sure why he would say this is easy? You could be right and he could be wrong and if so maybe you should be the CMO. But, I got to tell you, my money is on him. He will need more for the T2s but he should at least get a mention of inhaled insulin. In 18 months he can close the deal on the T2s but he should be able to close the T1 deal in 6 months along with the T2 mention. You realize that AFFINITY-1 and 171 are different names for the same trial? Equally that A1c was reduced by Afrezza in that trial on average by 0.21 to 7.77 and not 6.8. Today compliant Afrezza users are those who take a single dose at meal time. If that is to be changed it will take trial data, STAT-2 would be a prime candidate for that vehicle. I do think Dr Kendall can get inhaled insulin into the Type 1 recommendations by year end. I am curious to see if the ADA add inhaled insulin, or generalize the recommendation by removing the reference to injections. The reference documents for the trial are separate so its better to speak of them separate so not to confuse. Now, you can argue with Dr. Kendall but based on his analysis of the Affinty-1 www.ncbi.nlm.nih.gov/pubmed/26180109 here is what he is going to tell the ADA to get afrezza as the standard of care for T1s. Basically A1c 8.0 to 6.8 with no additional hypos.
You can also argue the trial was not large enough but it was good enough for FDA approval of afrezza. It had over 500 patients. You can also argue he can't get it done. He says its the easiest job he has ever had. One of you is right and the other is wrong. The target is January 2019. My money is on him, nothing personal.
- Use of Afrezza significantly lowers the rate of hypoglycemia in Type 1 diabetes while providing similar or better glycemic control (54.1 events per subject vs. 78.2 events per subject, a reduction of 31%)
- On average, 26% lower rates of hypoglycemia were observed with Afrezza across the range of HbA1c levels, allowing the same degree of glycemic control with less hypoglycemia than insulin aspart. For example, a patient with an HbA1c of 8.0% on insulin aspart would experience the same rate of hypoglycemia (12.2 events per month) as a patient on Afrezza with an HbA1c of 6.8% (ΔHbA1c = -1.2%)
- Alternatively, patients with HbA1c of 6.8% on Afrezza would be estimated to experience 4 fewer hypoglycemic events per month than a similar patient on insulin aspart
|
|
|
|
Post by agedhippie on Jul 4, 2018 10:50:49 GMT -5
You realize that AFFINITY-1 and 171 are different names for the same trial? Equally that A1c was reduced by Afrezza in that trial on average by 0.21 to 7.77 and not 6.8. Today compliant Afrezza users are those who take a single dose at meal time. If that is to be changed it will take trial data, STAT-2 would be a prime candidate for that vehicle. I do think Dr Kendall can get inhaled insulin into the Type 1 recommendations by year end. I am curious to see if the ADA add inhaled insulin, or generalize the recommendation by removing the reference to injections. The reference documents for the trial are separate so its better to speak of them separate so not to confuse. Now, you can argue with Dr. Kendall but based on his analysis of the Affinty-1 www.ncbi.nlm.nih.gov/pubmed/26180109 here is what he is going to tell the ADA to get afrezza as the standard of care for T1s. Basically A1c 8.0 to 6.8 with no additional hypos.
You can also argue the trial was not large enough but it was good enough for FDA approval of afrezza. It had over 500 patients. You can also argue he can't get it done. He says its the easiest job he has ever had. One of you is right and the other is wrong. The target is January 2019. My money is on him, nothing personal.
- Use of Afrezza significantly lowers the rate of hypoglycemia in Type 1 diabetes while providing similar or better glycemic control (54.1 events per subject vs. 78.2 events per subject, a reduction of 31%)
- On average, 26% lower rates of hypoglycemia were observed with Afrezza across the range of HbA1c levels, allowing the same degree of glycemic control with less hypoglycemia than insulin aspart. For example, a patient with an HbA1c of 8.0% on insulin aspart would experience the same rate of hypoglycemia (12.2 events per month) as a patient on Afrezza with an HbA1c of 6.8% (ΔHbA1c = -1.2%)
- Alternatively, patients with HbA1c of 6.8% on Afrezza would be estimated to experience 4 fewer hypoglycemic events per month than a similar patient on insulin aspart
You are way over-thinking this. Dr Kendall doesn't need to talk about anything other than the non-inferiority result. That alone is sufficient to get inhaled insulin into Standard of Care along side RAA since it is an equivalent insulin treatment. I reread the Affinity paper. This gets nowhere because, as the paper admits, the hypo data is not "not statistically significant". Nowhere does it mention moving an HbA1c 8.0 to 6.8. Interestingly once you get below 6.5 there is no statistical difference between Afrezza and Novolog, however nearly twice as many Novolog users made it as Afrezza users (10 to 19). |
|
|
|
Post by peppy on Jul 4, 2018 12:53:09 GMT -5
The reference documents for the trial are separate so its better to speak of them separate so not to confuse. Now, you can argue with Dr. Kendall but based on his analysis of the Affinty-1 www.ncbi.nlm.nih.gov/pubmed/26180109 here is what he is going to tell the ADA to get afrezza as the standard of care for T1s. Basically A1c 8.0 to 6.8 with no additional hypos.
You can also argue the trial was not large enough but it was good enough for FDA approval of afrezza. It had over 500 patients. You can also argue he can't get it done. He says its the easiest job he has ever had. One of you is right and the other is wrong. The target is January 2019. My money is on him, nothing personal.
- Use of Afrezza significantly lowers the rate of hypoglycemia in Type 1 diabetes while providing similar or better glycemic control (54.1 events per subject vs. 78.2 events per subject, a reduction of 31%)
- On average, 26% lower rates of hypoglycemia were observed with Afrezza across the range of HbA1c levels, allowing the same degree of glycemic control with less hypoglycemia than insulin aspart. For example, a patient with an HbA1c of 8.0% on insulin aspart would experience the same rate of hypoglycemia (12.2 events per month) as a patient on Afrezza with an HbA1c of 6.8% (ΔHbA1c = -1.2%)
- Alternatively, patients with HbA1c of 6.8% on Afrezza would be estimated to experience 4 fewer hypoglycemic events per month than a similar patient on insulin aspart
You are way over-thinking this. Dr Kendall doesn't need to talk about anything other than the non-inferiority result. That alone is sufficient to get inhaled insulin into Standard of Care along side RAA since it is an equivalent insulin treatment. I reread the Affinity paper. This gets nowhere because, as the paper admits, the hypo data is not "not statistically significant". Nowhere does it mention moving an HbA1c 8.0 to 6.8. Interestingly once you get below 6.5 there is no statistical difference between Afrezza and Novolog, however nearly twice as many Novolog users made it as Afrezza users (10 to 19). well aged, you know it is because the Novolog people had more hypos. We even see when it happens. At night, when insulin has been stacked all day and basal given? Did the HbA1c calculation come from the STAT? a 30mg/dl reduction average = 1 % point HbA1c. |
|
|
|
Post by agedhippie on Jul 4, 2018 16:04:37 GMT -5
You are way over-thinking this. Dr Kendall doesn't need to talk about anything other than the non-inferiority result. That alone is sufficient to get inhaled insulin into Standard of Care along side RAA since it is an equivalent insulin treatment. I reread the Affinity paper. This gets nowhere because, as the paper admits, the hypo data is not "not statistically significant". Nowhere does it mention moving an HbA1c 8.0 to 6.8. Interestingly once you get below 6.5 there is no statistical difference between Afrezza and Novolog, however nearly twice as many Novolog users made it as Afrezza users (10 to 19). well aged, you know it is because the Novolog people had more hypos. We even see when it happens. At night, when insulin has been stacked all day and basal given? Did the HbA1c calculation come from the STAT? a 30mg/dl reduction average = 1 % point HbA1c.
Stacking doesn't work like that. This is a decay curve for Novolog and Fiasp from the Loop AP project: 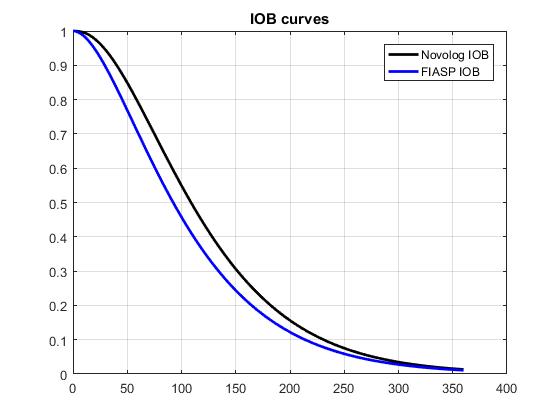 Basically anything I take will follow that curve in leaving my body. When I take insulin I deduct (or rather my bolus wizard does) any insulin remaining in me from the required dose. Stacking is not the danger, it's even desirable, overdosing is the danger. As an example assume I took 10u at 1pm and then ate something 3 hours later requiring 4u of insulin. Using the black Novolog curve I would have cleared about 80% of that original 10u. I would deduct 20% of the original bolus (2u) from the required 4u of insulin leaving 2u which is what I would take. I stacked insulin, I did not overdose, I can sleep soundly. Now about night time hypos. For me at least most of them are caused by the 2pm dip. Between 2pm and 3:30pm your body produces less basal insulin. If you are using an basal insulin then you need to make sure you have enough head room in your bed time level for that dip. If you go to bed at about 10pm and take Lantus it's a perfect storm because Lantus has a peak at about 5 hours which lands smack in the middle of that dip. I take Toujeo in the morning for exactly that reason. If you are on a pump you can do a far better job because you can reduce the insulin for that dip and increase it for the following pre-dawn spike. |
|
|
|
Post by agedhippie on Jul 4, 2018 16:36:54 GMT -5
You are way over-thinking this. Dr Kendall doesn't need to talk about anything other than the non-inferiority result. That alone is sufficient to get inhaled insulin into Standard of Care along side RAA since it is an equivalent insulin treatment. I reread the Affinity paper. This gets nowhere because, as the paper admits, the hypo data is not "not statistically significant". Nowhere does it mention moving an HbA1c 8.0 to 6.8. Interestingly once you get below 6.5 there is no statistical difference between Afrezza and Novolog, however nearly twice as many Novolog users made it as Afrezza users (10 to 19). well aged, you know it is because the Novolog people had more hypos. We even see when it happens. At night, when insulin has been stacked all day and basal given? Did the HbA1c calculation come from the STAT? a 30mg/dl reduction average = 1 % point HbA1c. Now to the question of whether the Novolog users go their result by a ton of hypos. There were 19 Novolog users with sub-6.5 A1c results (and 10 Afrezza users). Both Afrezza and Novolog users had a similar mild hypo rate (13 vs. 16 per subject month). That result is unsurprising since it's really easy to get a minor hypo and you often see it in the CGM graphs that get posted. A mild hypo will last tops an hour and more likely half an hour.Take the worst case of an hour and you are looking at 0.03% of the time in a minor hypo, that's not going to register in an HbA1c. Lets take the severe hypo and these can be anything up to 3 hours to fix, unless you get a glucagon shot in which case if it's intravenous (that's usually an EMT) it's a minute, and if it's intramuscular it's about five minutes. Lets say 3 hours as a worst case in which case you are looking at 0.18 incidences per month which gives 0.001% of the time which definitely is not going to impact the HbA1c. HbA1c is impacted by common longer lasting events - extended elevated numbers. For example today is a trainwreck, I went over 180 at about 11:30am and only got back at 4pm (I was being lazy with boluses). My TIR today is 63% so far, and SD is 36% which is ok, but my average is 173. If that happens repeatedly that is the sort of thing that hits HbA1c results. It is also a good example why TIR is not everything! On the "hmm" list; in the Affinity-1 paper that the ADA hypo results came from Novolog users with an HbA1c of 6.5 to 7.0, and 7.5 to 8.0 had essentially the same severe hypo rate as Afrezza users (Novolog users with HbA1c of 6.5 to 7.0 had marginally more hypos, and with 7.5-8.0 had marginally less). |
|
|
|
Post by goyocafe on Jul 4, 2018 16:43:55 GMT -5
well aged, you know it is because the Novolog people had more hypos. We even see when it happens. At night, when insulin has been stacked all day and basal given? Did the HbA1c calculation come from the STAT? a 30mg/dl reduction average = 1 % point HbA1c.
Stacking doesn't work like that. This is a decay curve for Novolog and Fiasp from the Loop AP project:  ustments. Basically anything I take will follow that curve in leaving my body. When I take insulin I deduct (or rather my bolus wizard does) any insulin remaining in me from the required dose. Stacking is not the danger, it's even desirable, overdosing is the danger. As an example assume I took 10u at 1pm and then ate something 3 hours later requiring 4u of insulin. Using the black Novolog curve I would have cleared about 80% of that original 10u. I would deduct 20% of the original bolus (2u) from the required 4u of insulin leaving 2u which is what I would take. I stacked insulin, I did not overdose, I can sleep soundly. Now about night time hypos. For me at least most of them are caused by the 2pm dip. Between 2pm and 3:30pm your body produces less basal insulin. If you are using an basal insulin then you need to make sure you have enough head room in your bed time level for that dip. If you go to bed at about 10pm and take Lantus it's a perfect storm because Lantus has a peak at about 5 hours which lands smack in the middle of that dip. I take Toujeo in the morning for exactly that reason. If you are on a pump you can do a far better job because you can reduce the insulin for that dip and increase it for the following pre-dawn spike. Semantics. The odds of an overdose increase with IOB since it is another variable in the equation. The sooner IOB is no longer a variable, the less chance of miscalculating proper dosing. The longer insulin takes to clear your system, the more time is spent calculating dose adjustments. Just applying simple logic. I’m sure there’s some philosopher that has articulated a statement about complex equations that would put my attempt to shame. |
|