|
|
Post by peppy on Aug 8, 2021 14:52:06 GMT -5
Wait — there's more! FTA:
"NRx Pharmaceuticals has entered a memorandum of understanding (MoU) with the Government of Israel to licence exclusive global development, manufacturing and commercialisation rights to a Covid-19 vaccine, BriLife."
"Developed by the Israel Institute for Biological Research (IIBR), BriLife is based on a vaccine platform previously approved by the US Food and Drug Administration (FDA)."
"NRx chairman and CEO Jonathan Javitt along with the company’s director Chaim Hurvitz, who chairs an Israeli private equity group, CH Health, will lead the development initiative."
"Hurvitz said: “As the first generation Covid vaccines are increasingly challenged by rapid mutation of the coronavirus, we aim to develop a vaccine that can rapidly scale at low cost to serve the needs of both the developed and the developing world.”"
"As BriLife is a self-propagating, live-virus vaccine, NRx expects that it can be quickly and cost-effectively scaled up and produced in industrial settings. Initially, the vaccine will be administered via standard injection."
After initial use, could the end goal be an inhaled Technosphere vaccine? Now wouldn't that make Al Mann [RIP] smile?
“ This linkage between the clinical effect of aviptadil on survival and recovery and a measurable biologic change in cytokine levels provides a basis for seeking a biomarker-based regulatory path as envisioned by the 21st Century Cures Act.”The company submitted the data from the trial to the US Food and Drug Administration (FDA) to support its Emergency Use Authorization (EUA) application for Zyesami to treat critically ill Covid-19 patients. Furthermore, NRx is planning to submit a biomarker letter of intent to the FDA based on these Phase IIb/III results. The latest development comes after NRx entered a memorandum of understanding (MoU) with the Government of Israel to license exclusive global development, manufacturing and commercialisation rights to a Covid-19 vaccine, BriLife.  Results: Nineteen of 21 patients survived to day 28 in the aviptadil-treated group compared to 4 of 24 control patients (90% vs 17%; P<.0001). poseidon01.ssrn.com/delivery.php?ID=266082064082025028088016100087012000101015002033002030090080112006071086106064018007029026017010047102021127110007028067108002104082030064006069089028127073101083096022035041112081021122092009096091121096089002112077066121003088095071004098001026126098&EXT=pdf&INDEX=TRUEclinicaltrials.gov/ct2/show/NCT04360096Inhaled ZYESAMI™ (Aviptadil Acetate) for the Treatment of Severe COVID-19 (AVICOVID-2) Device: Nebulized administration of ZYESAMI™ or Placebo |
|
|
|
Post by peppy on Aug 8, 2021 18:39:24 GMT -5
|
|
|
|
Post by goyocafe on Aug 8, 2021 18:53:34 GMT -5
This is for a nebulized version I believe. The more this moves in the direction of an inhaled DPI formulation, the better. What I’m hoping for is the inhaled version can be used prophylacticly at the first sign of symptoms and bring hospitalizations to a minimum. |
|
|
|
Post by peppy on Aug 8, 2021 19:09:21 GMT -5
This is for a nebulized version I believe. The more this moves in the direction of an inhaled DPI formulation, the better. What I’m hoping for is the inhaled version can be used prophylacticly at the first sign of symptoms and bring hospitalizations to a minimum. Nebulizer, The earnings report is coming up.... I wonder if MNKD has it formulated and if the phase one dosing trial is on it's way? Is that the line up now? Added, anyway, Fauci and NRXP knows the medication needs to get into the alveoli where the ACEII receptors are located for the surfactant and then the blood stream. "As soon as surfactant is gone, you are in profound respiratory failure and that's what kills people in Covid19." Dr Jonathan Javitt of NeuroRx Pharma.
an oldie but goodie. and they know right where they want the Aviptadil to go.  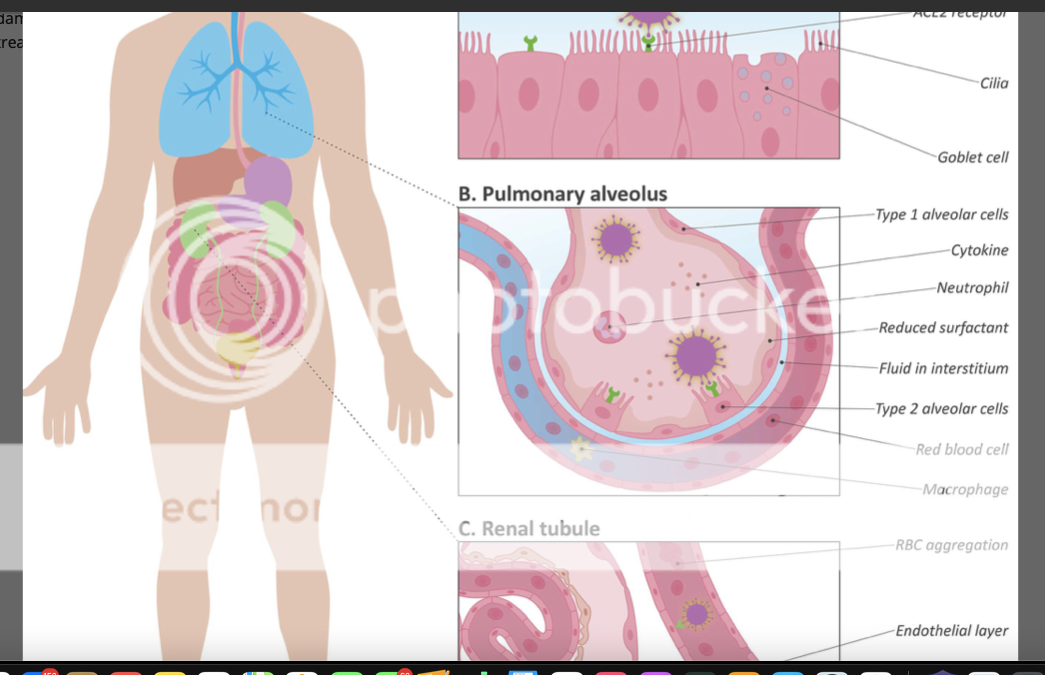 mango mango |
|
|
|
Post by peppy on Aug 8, 2021 21:03:18 GMT -5
Wait — there's more! FTA:
"NRx Pharmaceuticals has entered a memorandum of understanding (MoU) with the Government of Israel to licence exclusive global development, manufacturing and commercialisation rights to a Covid-19 vaccine, BriLife."
"Developed by the Israel Institute for Biological Research (IIBR), BriLife is based on a vaccine platform previously approved by the US Food and Drug Administration (FDA)."
"NRx chairman and CEO Jonathan Javitt along with the company’s director Chaim Hurvitz, who chairs an Israeli private equity group, CH Health, will lead the development initiative."
"Hurvitz said: “As the first generation Covid vaccines are increasingly challenged by rapid mutation of the coronavirus, we aim to develop a vaccine that can rapidly scale at low cost to serve the needs of both the developed and the developing world.”"
"As BriLife is a self-propagating, live-virus vaccine, NRx expects that it can be quickly and cost-effectively scaled up and produced in industrial settings. Initially, the vaccine will be administered via standard injection."
After initial use, could the end goal be an inhaled Technosphere vaccine? Now wouldn't that make Al Mann [RIP] smile?
The explanation at. the 44 min mark. www.youtube.com/watch?v=Myt-KgPBuPUparaphrasing, there is now a modified mouse that has ACE2 receptors and when given COVID19 will die. NRx, is contracted with Israel to test their new vaccine with the mouse model they have. Not sure the technical words are correct. |
|
|
|
Post by peppy on Aug 8, 2021 21:34:00 GMT -5
|
|
|
|
Post by mymann on Aug 8, 2021 21:58:54 GMT -5
Didn't mnkd have some sort of patent on VIP?
|
|
|
|
Post by goyocafe on Aug 8, 2021 22:38:21 GMT -5
|
|
|
|
Post by peppy on Aug 9, 2021 6:40:48 GMT -5
Didn't mnkd have some sort of patent on VIP? yes. Posted by Mango, I dug it up. ================================================================================================ Yes, MannKind already has patents covering Vasoactive Intestinal Peptide. Take this recent patent for example, granted August 25, 2020 (just one of several): 4. The medicament cartridge of claim 2, wherein the active ingredient is insulin, heparin, calcitonin, felbamate, sumatriptan, parathyroid hormone, growth hormone, erythropoietin, AZT, DDI, granulocyte macrophage colony stimulating factor, lamotrigine, chorionic gonadotropin releasing factor, luteinizing releasing hormone, beta-galactosidase, exendin, vasoactive intestinal peptide, follicle stimulating hormone, vasoactive intestinal peptide, parathyroid hormone, parathyroid hormone related protein, glucagon-like peptide-1, exendin, oxyntomodulin, peptide YY, interleukin 2-inducible tyrosine kinase, Bruton's tyrosine kinase, inositol-requiring kinase 1, PC-DAC-modified derivative, or O-glycosylated forms of PC-DAC, parathyroid hormone 1-34, argatroban, or a combination thereof. patents.justia.com/patent/10751488#claims |
|
|
|
Post by markado on Aug 9, 2021 11:17:52 GMT -5
Count'em up, folks, that's 31 different solutions listed under a granted patent as of 2020. And, we have approx. 10 of those in motion, with more potential partners coming out of the woodwork. We are WAAAAAAAY undervalued and really just getting started at the Platform technology level - Afrezza aside.
|
|
|
|
Post by MnkdWASmyRtrmntPlan on Aug 9, 2021 11:26:56 GMT -5
Count'em up, folks, that's 31 different solutions listed under a granted patent as of 2020. And, we have approx. 10 of those in motion, with more potential partners coming out of the woodwork. We are WAAAAAAAY undervalued and really just getting started at the Platform technology level - Afrezza aside. Keep accumulating!!!  |
|
|
|
Post by peppy on Aug 9, 2021 11:36:04 GMT -5
Count'em up, folks, that's 31 different solutions listed under a granted patent as of 2020. And, we have approx. 10 of those in motion, with more potential partners coming out of the woodwork. We are WAAAAAAAY undervalued and really just getting started at the Platform technology level - Afrezza aside. I think the NIH could be the partner from heaven. ir.nrxpharma.com/news-events/press-releases/detail/76/nrx-pharmaceuticals-partners-with-mannkind-corporation-to "said Prof Jonathan Javitt, MD, MPH, CEO and Chairman of NRx (see concept image). "I had the privilege of working closely with Dr. Alfred Mann, on the refinement and regulatory approval of MannKind's Technosphere platform and have long admired its simplicity and elegance. On many occasions he and I discussed his vision to extend Technosphere beyond insulin to solve the unique stability and administration challenges of peptide-based drugs. I am personally delighted to be partnering once again with MannKind and bringing Dr. Mann's vision to life." Also in the article(not MNKD formulation, but a nebulizer) "Both intravenous and inhaled formulations of ZYESAMI™ are in phase 3 clinical trials funded by the US National Institutes of Health, the Biomedical Advanced Research Development Authority (BARDA), and by NRx." Oh yeah. Detailed Description: This is a master protocol to evaluate the safety and efficacy of investigational agents aimed at improving outcomes for patients with acute respiratory failure related to COVID-19. Trials within this protocol will be adaptive, randomized, blinded and initially placebo-controlled. Participants will receive standard of care (SOC) treatment as part of the protocol. If an investigational agent shows superiority over placebo, SOC for the study of future investigational agents may be modified accordingly.The international trials within this protocol will be conducted in up to several hundred clinical sites. Participating sites are affiliated with networks funded by the United States National Institutes of Health (NIH) and the US Department of Veterans Affairs. clinicaltrials.gov/ct2/show/NCT04843761?term=NCT04843761&draw=2&rank=1This is the nebulizer trial. and the results... of the IV doses, Biological: Aviptadil Administered by IV infusion over 12 hours per day for 3 days. because I am ad nauseam and I am excited.   "Based on these findings, aviptadil has been selected for inclusion in the Corona Virus Tech Watch Program administered by the US Department of Health and Human Services. COVID-19 therapeutics will remain a top priority, no matter how promising the efforts at vaccine development become. Unlike expensive-to-produce monoclonal antibodies and difficult-to-scale convalescent plasma, VIP can ultimately be produced at a cost of pennies per dose, if moved from chemical peptide synthesis to yeast fermentation, as is done for insulin. As such, it may represent a critical therapeutic for COVID-19 and possibly other viral infections for both the developed and the developing world." poseidon01.ssrn.com/delivery.php?ID=266082064082025028088016100087012000101015002033002030090080112006071086106064018007029026017010047102021127110007028067108002104082030064006069089028127073101083096022035041112081021122092009096091121096089002112077066121003088095071004098001026126098&EXT=pdf&INDEX=TRUE the last paragraph, page 11 |
|
|
|
Post by peppy on Aug 9, 2021 12:31:18 GMT -5
Hold the bus..... "Based on these findings, aviptadil has been selected for inclusion in the Corona Virus Tech Watch Program administered by the US Department of Health and Human Services. COVID-19 therapeutics will remain a top priority, no matter how promising the efforts at vaccine development become. Unlike expensive-to-produce monoclonal antibodies and difficult-to-scale convalescent plasma, VIP can ultimately be produced at a cost of pennies per dose, if moved from chemical peptide synthesis to yeast fermentation, as is done for insulin. As such, it may represent a critical therapeutic for COVID-19 and possibly other viral infections for both the developed and the developing world." poseidon01.ssrn.com/delivery.php?ID=266082064082025028088016100087012000101015002033002030090080112006071086106064018007029026017010047102021127110007028067108002104082030064006069089028127073101083096022035041112081021122092009096091121096089002112077066121003088095071004098001026126098&EXT=pdf&INDEX=TRUE the last paragraph, page 11 ======================================================================================================== I saw Lilly, From Kevin on ST, according to this, Eli Lilly owns 11.72% of UTHR. One big happy family  . www.wallstreetzen.com/stocks/us/nasdaq/uthr/ownership======================================================================================================= Who has the E.Coli fermenter? Lilly.  Just sayin. |
|
|
|
Post by goyocafe on Aug 10, 2021 6:25:31 GMT -5
NOW would be a good time to announce successful formulation of Techno-VIP.
|
|
|
|
Post by veritasfiliatemporis on Aug 10, 2021 13:29:59 GMT -5
Did I hear the pop!!! uhmmm waiting the sixties ..
|
|










 .
.