|
|
Post by buyitonsale on Jan 18, 2022 16:12:52 GMT -5
I like the news today, so I have added some more shares to celebrate.
We will find out FDA decision 4 months sooner than previously estimated by UTHR.
|
|
|
|
Post by mytakeonit on Jan 18, 2022 16:17:11 GMT -5
nxc2 ... boca will want a dinner to go along with that drink.  But, that's mytakeonit |
|
|
|
Post by cretin11 on Jan 18, 2022 17:09:19 GMT -5
One can surely continue to hope. For the long-timers here, I used the user name "Q2U" since I started and long before QAnon appeared. It was based on Star Trek, without any connection to this political/cultural group. So for clarity, I have changed my user name and continue to hold long on MNKD and lurk here accordingly. GLTA. Welcome, and can’t blame you for the name change, smart move! |
|
|
|
Post by BlueCat on Jan 19, 2022 15:10:40 GMT -5
One can surely continue to hope. For the long-timers here, I used the user name "Q2U" since I started and long before QAnon appeared. It was based on Star Trek, without any connection to this political/cultural group. So for clarity, I have changed my user name and continue to hold long on MNKD and lurk here accordingly. GLTA. Welcome, and can’t blame you for the name change, smart move! Many thanks Cretin et all. I check in on y'all routinely and to get your takes on the various developments. Suffice to say, I share the ongoing exasperation with the longs here - both on the market control over the stock, and the challenge of breaking through to help diabetics. I'm glad there is better diversification/traction in the product lines - especially with Tyvaso near term. I do wish MNKD had more success on their RTM (route-to-market) diversification - more success in those overseas markets. More partnerships like VDEX. Funny how some things have come full circle. I really hope we finally break through over these next months. Standing by in any case. |
|
|
|
Post by prcgorman2 on Jan 20, 2022 7:47:25 GMT -5
I didn’t know what “user fee goal date” was that was mentioned in the UTHR 8-K and in the press release(s). I found a couple of FDA documents that described the term and regulations on responses to NDA filing after a CRL. The “user fee goal date” is the deadline for the FDA to respond to the refile. If I understood correctly, by regulation it is within 2 months of the refiling which would put it in late February at the latest. I also learned that the FDA regulation for the timeline for them to acknowledge a refiling had been 14 (or maybe it was 15) days, but later changed to 30 days. And, I also learned that there are two classes of FDA requirements for review; Class 1 and Class 2. Those have been mentioned on this board. The definition is kind of interesting. Basically, there is a laundry list of items which are Class 1, and then the definition of Class 2 is whatever isn’t Class 1. That amused me. Anyway, the layman’s version of Class 1 might be “minor” and Class 2 as “major”. The UTHR NDA is Class 1. Another interesting tidbit in that classification is the FDA regulation indicates Class 1 does not require reinspection. I wondered about that because of the inspection issue that caused the PDUFA not to be approved in the CRL from the FDA. And, who knows? Maybe there was a reinspection anyway even if Class 1 did not require it. In any case, it looks promising, and of course the consensus on the street is the same. UTHR is trading near an all-time high, and MNKD managed to eek out gain on one of the worst days on the Street and the NASDAQ.
I’m happy to report I added under $4, and I may feather the holdings a little more. I hit my basic goal of holdings some time ago but couldn’t resist adding the last 2 (or is it 3?) times the MNKD share price dipped below $4. Is that greed, or is that smart? Could be both I suppose!
|
|
|
|
Post by cppoly on Jan 20, 2022 8:47:57 GMT -5
Does anyone have any statistics on Class 1 approvals?
What percent are approved?
How is this percent compared to Class 2?
I'd assume Class 1 should be GREAT news compared to Class 2 but the SP is not acting this way. Is it just typical irrational behavior? Can this behavior be calmed down with real statistics?
Shaving 4 months off approval is amazing. In addition, the FDA cited no safety concerns and only indicated a minor inspection issue. This should be a homerun and I'm sure UTHR has resolved the issue. Am I missing something here??
|
|
|
|
Post by liane on Jan 20, 2022 8:55:02 GMT -5
I personally think the op ex tomorrow is holding this down.
|
|
|
|
Post by kenken on Jan 20, 2022 9:46:48 GMT -5
Did I inform folks about LQDA when it was around $2.50 area? Stand your foot in the ground, no feet. Watch out Leap of Feb 18.
|
|
|
|
Post by peppy on Jan 20, 2022 9:57:54 GMT -5
Did I inform folks about LQDA when it was around $2.50 area? Stand your foot in the ground, no feet. Watch out Leap of Feb 18. True. May I put it in the atmosphere, that pigs may be slaughtered. 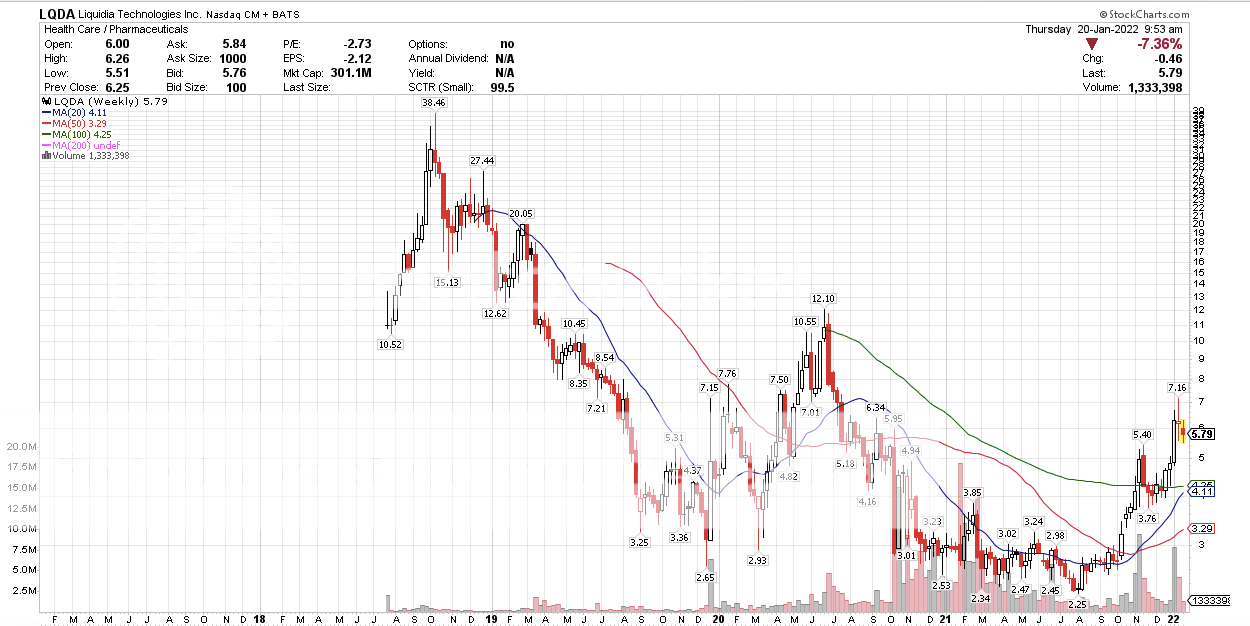 |
|
rebby
Researcher
  
Posts: 79
|
Post by rebby on Jan 20, 2022 10:12:53 GMT -5
Did I inform folks about LQDA when it was around $2.50 area? Stand your foot in the ground, no feet. Watch out Leap of Feb 18. What is the value of the Feb 18 Leap? |
|
|
|
Post by celo on Jan 20, 2022 10:14:51 GMT -5
I personally think the op ex tomorrow is holding this down. Amazing how obvious they make it. |
|
|
|
Post by cretin11 on Jan 20, 2022 10:48:36 GMT -5
I personally think the op ex tomorrow is holding this down. Amazing how obvious they make it. Like clockwork. |
|
|
|
Post by prcgorman2 on Jan 20, 2022 13:21:25 GMT -5
Does this means we finally have a reason to look forward to a Monday?
|
|
|
|
Post by liane on Jan 20, 2022 14:02:29 GMT -5
Amazing how obvious they make it. Like clockwork. Again! |
|
|
|
Post by mymann on Jan 20, 2022 17:31:52 GMT -5
So 2 months review instead of 6 months review, market treating this good news as if we're not going to get approval or are they trying to shake the tree for weak hands before for the sp goes up? Anyone?
|
|