|
|
Post by bones1026 on Apr 6, 2022 22:44:28 GMT -5
It means between now and the end of May. I agree that it could be approved even before May. I see that Mannkind tweeted out these results as well. Yet we heard nothing from them when RLS had a PR? This almost feels trying to get ahead of bad FDA news…again. |
|
|
|
Post by hellodolly on Apr 7, 2022 5:46:37 GMT -5
I agree that it could be approved even before May. I see that Mannkind tweeted out these results as well. Yet we heard nothing from them when RLS had a PR? This almost feels trying to get ahead of bad FDA news…again. Three things that I'm pondering as I read your post: 1. It's an announcement of the journal publication and the contents of the peer review, just published two weeks ago. They are not rehashing any old news. 2. RLS isn't even at the cusp of any drug approvals, unlike UTHR. UTHR is very invested in this drug. 3. MNKD should Tweet, they have a lot of skin in the game, too. |
|
|
|
Post by anderson on Apr 7, 2022 6:08:28 GMT -5
Just cant logically see how the FDA will be able to deny Tyvaso DPI. If they are corrupt I can see them saying more testing is needed to push back approval, but really the delivery system has been tested for years with Afrezza, and inhaled Tyvaso has been around for years as well. Putting the two together should be as simple as showing they don't react on one another.
|
|
|
|
Post by prcgorman2 on Apr 7, 2022 6:52:34 GMT -5
I agree that it could be approved even before May. I see that Mannkind tweeted out these results as well. Yet we heard nothing from them when RLS had a PR? This almost feels trying to get ahead of bad FDA news…again. I would kind of wonder about the wisdom of drawing attention if you thought you were going to get shot down. Even so, I also wondered if UTHR was rubbing noses in information as a way of saying, “we’ve got a lot of information we can use if this turns into a fight”. I have never tried to learn what recourse is available to a company that is denied approval, and rubbing a regulator’s nose in information seems kind of sophmoric unless it really does somehow improve a legal position. Dr. Rothblatt was a very successful attorney working with the FCC to create satellite radio, so whatever the reason for the publication, if Dr. Rothblatt had a say in it, I trust her judgement. |
|
|
|
Post by Chris-C on Apr 7, 2022 17:45:44 GMT -5
Harryx1 wrote:
Currently sitting at ~6 month delay from @us_FDA @drcaliff_FDA so far for Tyvaso DPI due to an erroneous CP on Technosphere/FDKP that is used in another FDA approved drug & has over 125,000 Rxs filled w/no safety issues. A travesty for ill PAH patients!
------------------------------------
@theharryx1:
I think use of the term "erroneous citizen Petition" is being generously kind. It's widely recognized that the CP is a method abused for anti competitive reasons. If FDA is concerned about the abuse of the process, why are they delaying approvals and using that as an excuse? That reinforces abuse of the process, or don't they realize that???
IMO, Some new Citizen Petition rules are in order:
1). CP's cannot be filed without disclosing their source.
2) Erroneous filings that delay approvals should have consequences. Filings that result from avoidable misinterpretations of existing data or failure to consider or be aware of data publicly available should result in sanctions. Suspension of CP filing privileges comes to mind.
3) Companies affected by erroneous or trivial filings that result in delayed approvals should be entitled to seek damages from authors of the petitions.
4 The burden of proof should be the responsibility of the CP filer. Neither the FDA nor the NDA applicant should be burdened with disproving safety concerns arising from CPs.
-Chris C
|
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Apr 7, 2022 19:14:52 GMT -5
Harryx1 wrote:Currently sitting at ~6 month delay from @us_FDA @drcaliff_FDA so far for Tyvaso DPI due to an erroneous CP on Technosphere/FDKP that is used in another FDA approved drug & has over 125,000 Rxs filled w/no safety issues. A travesty for ill PAH patients! ------------------------------------ @theharryx1: I think use of the term "erroneous citizen Petition" is being generously kind. It's widely recognized that the CP is a method abused for anti competitive reasons. If FDA is concerned about the abuse of the process, why are they delaying approvals and using that as an excuse? That reinforces abuse of the process, or don't they realize that??? IMO, Some new Citizen Petition rules are in order: 1). CP's cannot be filed without disclosing their source. 2) Erroneous filings that delay approvals should have consequences. Filings that result from avoidable misinterpretations of existing data or failure to consider or be aware of data publicly available should result in sanctions. Suspension of CP filing privileges comes to mind. 3) Companies affected by erroneous or trivial filings that result in delayed approvals should be entitled to seek damages from authors of the petitions. 4 The burden of proof should be the responsibility of the CP filer. Neither the FDA nor the NDA applicant should be burdened with disproving safety concerns arising from CPs. -Chris C The CP played into the FDA's plan. MISSION ACCOMPLISHED!!! The FDA hates to give a pharmaceutical company unabated free will. FDA was pissed with UTHR for their lawsuit with LQDA. First UTHR spent $105M on the PRV.....That went out the window with the weak "technical" reason for the first delay. Really??? A third party testing facility for trepostinil that did not cause any stoppage in production of the drug? That's strange!!! And now they waited 3 more months to bring up the Citizens Petition??? why couldn't they have requested the safety documents 9 months ago when the CP was filed? When you connect the DOTS you will see a 12-18 month DELAY which gets you closer to the end of the litigation HOLDING PERIOD between LQDA v UTHR. Interesting isn't it?  |
|
|
|
Post by cretin11 on Apr 7, 2022 21:47:00 GMT -5
Why was FDA pissed at UTHR about the litigation vs LQDA?
|
|
|
|
Post by buyitonsale on Apr 7, 2022 23:17:14 GMT -5
Not "pissed about lawsuit with LQDA", most likely paid by LQDA.
When I saw the first CRL and the mention of CP (not being reviewed at this time), my gut feeling was that they left another reason to delay at a later time. And I am sure many others here felt the same way...
That is exactly what happened.
I am mentally prepared for another CRL in May. As an investor I have to be ready for anything...
But I feel sorry for patients that are currently suffering from PAH and are not able to access better treatment options because of corruption.
|
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Apr 8, 2022 8:01:49 GMT -5
Why was FDA pissed at UTHR about the litigation vs LQDA? The FDA wants competition between drug companies. They want price competition. If you have ever noticed similar competitive drugs get approved around the same time. That's by design. The FDA did not like how the 800 Lb Gorilla sued LQDA and then bought the PRV. UTHR had every right to do what they did and would have had an 18 month lead over LQDA. They knew Tyvaso DPI would have a HUGE advantage over LQDA's Trepostinil. Tyvaso makes $600M a year in revenue and will get to a BILLION pretty quickly without DPI. The numbers are staggering and the FDA realize this and are delaying the DPI. |
|
|
|
Post by cretin11 on Apr 8, 2022 9:05:24 GMT -5
Ok thanks for explaining. There are a lot of dots to connect and that one is predicated on FDA siding against UTHR in favor of little ole LQDA. Which is interesting because usually on this message board we hear about FDA siding with the big gorillas against little MNKD. Who knows.
|
|
|
|
Post by radgray68 on Apr 8, 2022 10:56:13 GMT -5
FDA is proving to be either corrupt or inept. Neither are acceptable.
Try another delay and it's time to "Go to the mattresses" with these fools. Judging by the language she's used, I think Martine's prepared.
|
|
|
|
Post by peppy on Apr 8, 2022 11:01:17 GMT -5
FDA is proving to be either corrupt or inept. Neither are acceptable. Try another delay and it's time to "Go to the mattresses" with these fools. Judging by the language she's used, I think Martine's prepared. Perhaps the work still has yet to be done, and the student/FDA writes the note, the dog ate my homework? |
|
|
|
Post by biffn on Apr 8, 2022 11:12:24 GMT -5
So how are we going down since this news came out? Is this sell on the news or is there more to it? UTHR is up 3% since the news.
|
|
|
|
Post by peppy on Apr 8, 2022 11:21:07 GMT -5
So how are we going down since this news came out? Is this sell on the news or is there more to it? UTHR is up 3% since the news.What? exactly are you saying? 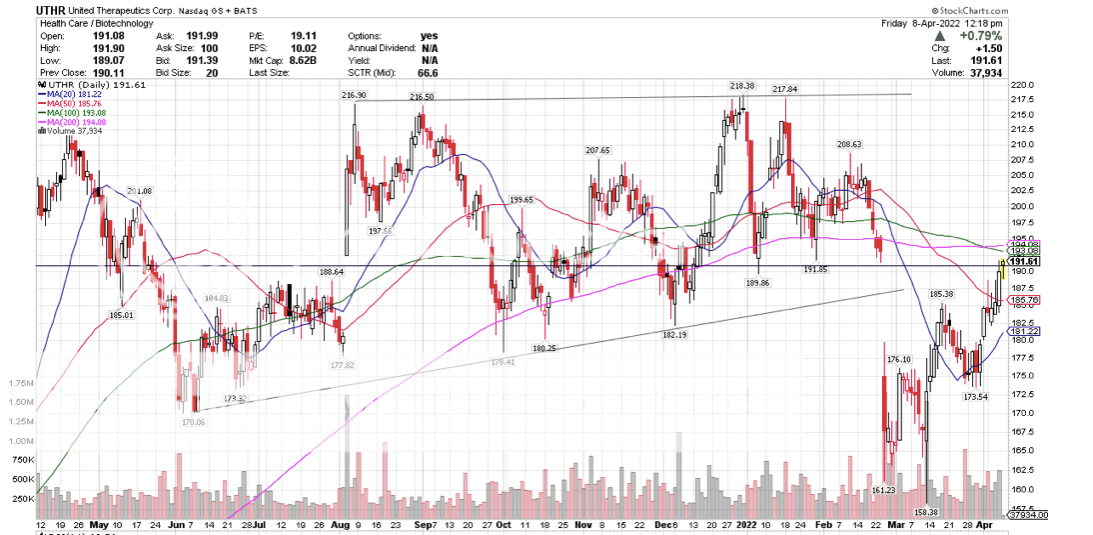 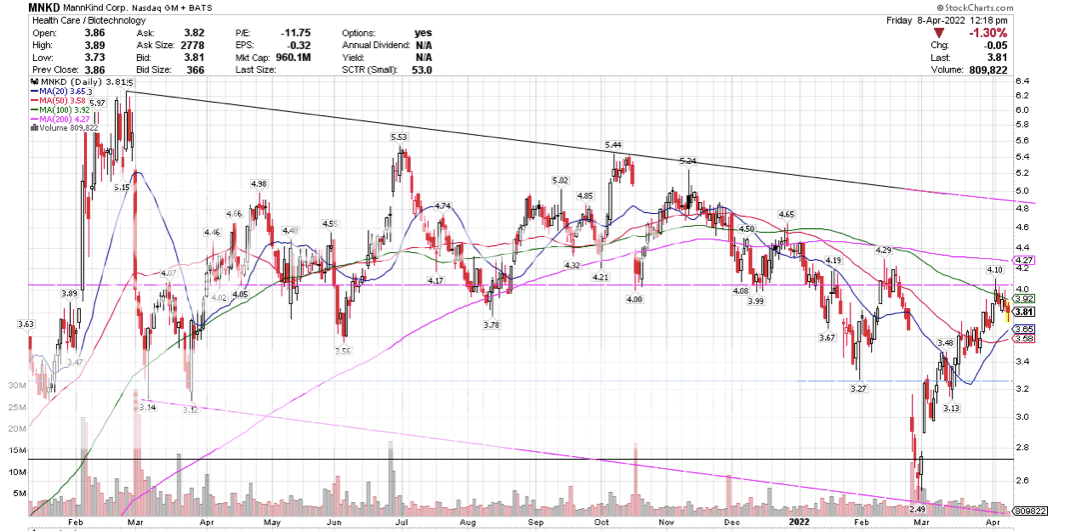 |
|
|
|
Post by biffn on Apr 8, 2022 13:56:23 GMT -5
Peppy,
UTHR announced the findings before the open on April 6, and has been up every day since. MNKD has been down every day since.
|
|