|
|
Post by harryx1 on May 23, 2022 12:53:20 GMT -5
|
|
|
|
Post by veritasfiliatemporis on May 23, 2022 12:55:38 GMT -5
And the Winner is harryx1
|
|
|
|
Post by boca1girl on May 23, 2022 12:56:51 GMT -5
Does closed means dismissed without taking any action? |
|
|
|
Post by avi8torslc on May 23, 2022 12:58:35 GMT -5
PPS could be a pretty good indicator
|
|
|
|
Post by joshuabohinc on May 23, 2022 12:59:06 GMT -5
download report here. www.regulations.gov/document/FDA-2021-P-0714-0008Conclusion: the FDA agency does not believe additional pre-market safety data were required to support the approval of NDA 214324 for Tyvaso DPI. Any potential pulmonary risks of Tyvaso DPI are adequately characterized by the available date and appropriately addressed and mitigated through product labeling. Further, we do not believe a contraindiciation, boxed warning, or REMS should be included in product labeling. Consequently, the Petition is denied. |
|
|
|
Post by harryx1 on May 23, 2022 12:59:51 GMT -5
III. CONCLUSION
As discussed above, the Agency does not believe additional pre-market safety data were required
to support the approval of NDA 214324 for Tyvaso DPI. Any potential pulmonary risks of
Tyvaso DPI are adequately characterized by the available data and appropriately addressed and
mitigated through product labeling. Further, we do not believe a contraindication, boxed
warning, or REMS should be included in product labeling. Consequently, the Petition is denied.
|
|
|
|
Post by scottmnkd on May 23, 2022 12:59:56 GMT -5
I. CONCLUSION
As discussed above, the Agency does not believe additional pre-market safety data were required
to support the approval of NDA 214324 for Tyvaso DPI. Any potential pulmonary risks of
Tyvaso DPI are adequately characterized by the available data and appropriately addressed and
mitigated through product labeling. Further, we do not believe a contraindication, boxed
warning, or REMS should be included in product labeling. Consequently, the Petition is denied.
|
|
|
|
Post by scottmnkd on May 23, 2022 13:00:40 GMT -5
I. CONCLUSION As discussed above, the Agency does not believe additional pre-market safety data were required to support the approval of NDA 214324 for Tyvaso DPI. Any potential pulmonary risks of Tyvaso DPI are adequately characterized by the available data and appropriately addressed and mitigated through product labeling. Further, we do not believe a contraindication, boxed warning, or REMS should be included in product labeling. Consequently, the Petition is denied. Looks like I took 3rd place on this one.  |
|
|
|
Post by peppy on May 23, 2022 13:02:25 GMT -5
PPS could be a pretty good indicator open at $4.50. if it holds, goes another .40 ..... The close, 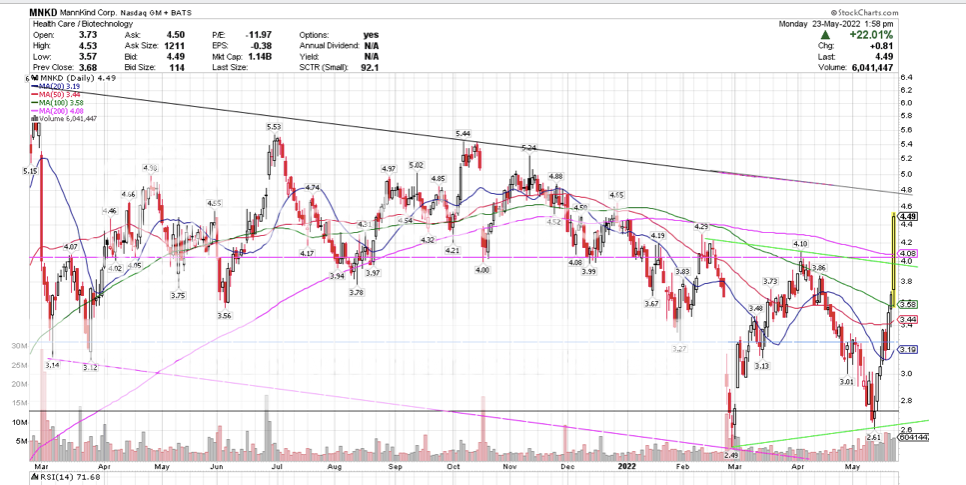 The open 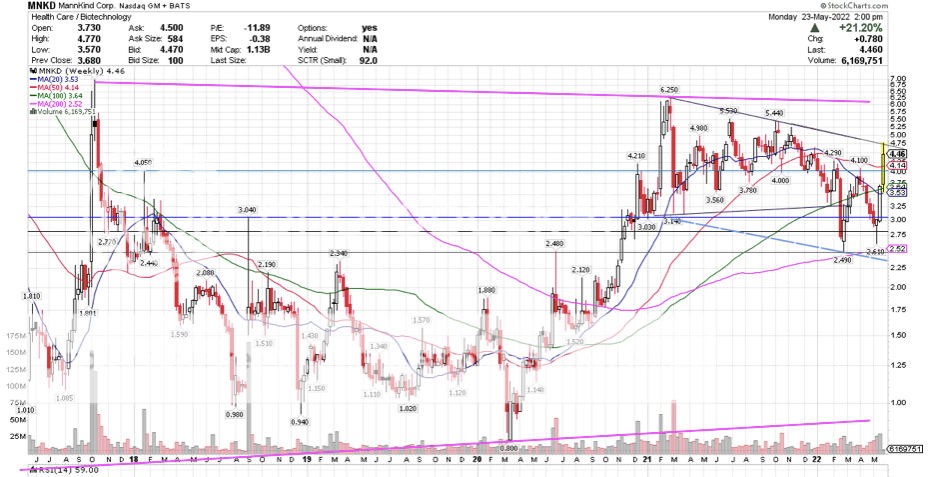 |
|
|
|
Post by porkini on May 23, 2022 13:04:40 GMT -5
LOL scottmnkd, hammer down like the FDA on Lassman.  |
|
|
|
Post by sayhey24 on May 23, 2022 13:04:41 GMT -5
WOW! A Clean label is coming and so is approval. Finally some great news!
|
|
|
|
Post by veritasfiliatemporis on May 23, 2022 13:04:52 GMT -5
Plus 22 M$ on top next quarter... Let's criticize MC, BTW I was worried about the black box, I think the path to get rid of that also for Afrezza should be paved...
|
|
|
|
Post by avi8torslc on May 23, 2022 13:06:10 GMT -5
DRUG GETS APPROVAL!
|
|
|
|
Post by factspls88 on May 23, 2022 13:06:41 GMT -5
As a long time mnkd bag holder, I’m not ready to pop open the. champagne but I’m very happy nonetheless. Great to hear that that there will be no boxed warning. I hope that this bodes well for the removal of Afrezza’s black box.
|
|
|
|
Post by cedafuntennis on May 23, 2022 13:08:56 GMT -5
Will UTHR and/MNKD file countersuit against the corrupt attorney who filled this frivolous suit and caused this delay and millions of dollars in lost revenues and put countless patients at risk? I would.
|
|