|
|
Post by jjacobs122 on Jun 1, 2022 11:31:08 GMT -5
Discussion of additional positive catalysts Pediatric phase 3 affreza testing….. Not till late this year or later
Receptor life Sciences cannabis product…… no definite timeline. Thoughts on when this may appear for approval?
Migraine headache treatment…… Thoughts on timeline?
Any further discussion is welcome |
|
|
|
Post by phdedieu12 on Jun 1, 2022 17:16:03 GMT -5
Approval?? I think your question isn't very clear. RLS is moving forward with a product, so it seems like they're making progress on "something", but to discuss what that "something" is, how far along in the development it is and when it might be approved is pure speculation. The good news is that they're still working on it and they clearly see something worth working on. But since you ask, 2 years.
Spoke with Mike, the next steps are already known: getting as many Tyveso patients on DPI, push Clofazomine along, finish the studies on peds, continue to push Afrezza sales (looks like a positive uptrend so far), and if this works, more resources will be allocated to accelerate the process, get V-GO going. The rest is all pipeline, and as we know, it took 4 years to get Tyveso through the approval, so any new molecule won't be out until 2026
|
|
|
|
Post by sayhey24 on Jun 1, 2022 17:49:54 GMT -5
I think the next big catalyst will be an afrezza "sales" partnership but who knows. V-Go came out of left field. Getting as many Tyveso patients on DPI is UTHR's job. Mike's job is to make Tyvaso DPI and sell afrezza.
|
|
|
|
Post by peppy on Jun 1, 2022 17:59:56 GMT -5
Discussion of additional positive catalysts -Perhaps the approval in India. -Peds trial is Estimated Primary Completion Date : April 2023 Estimated Study Completion Date : April 2024 Migraine medicine is no longer on MNKDs pipeline list. 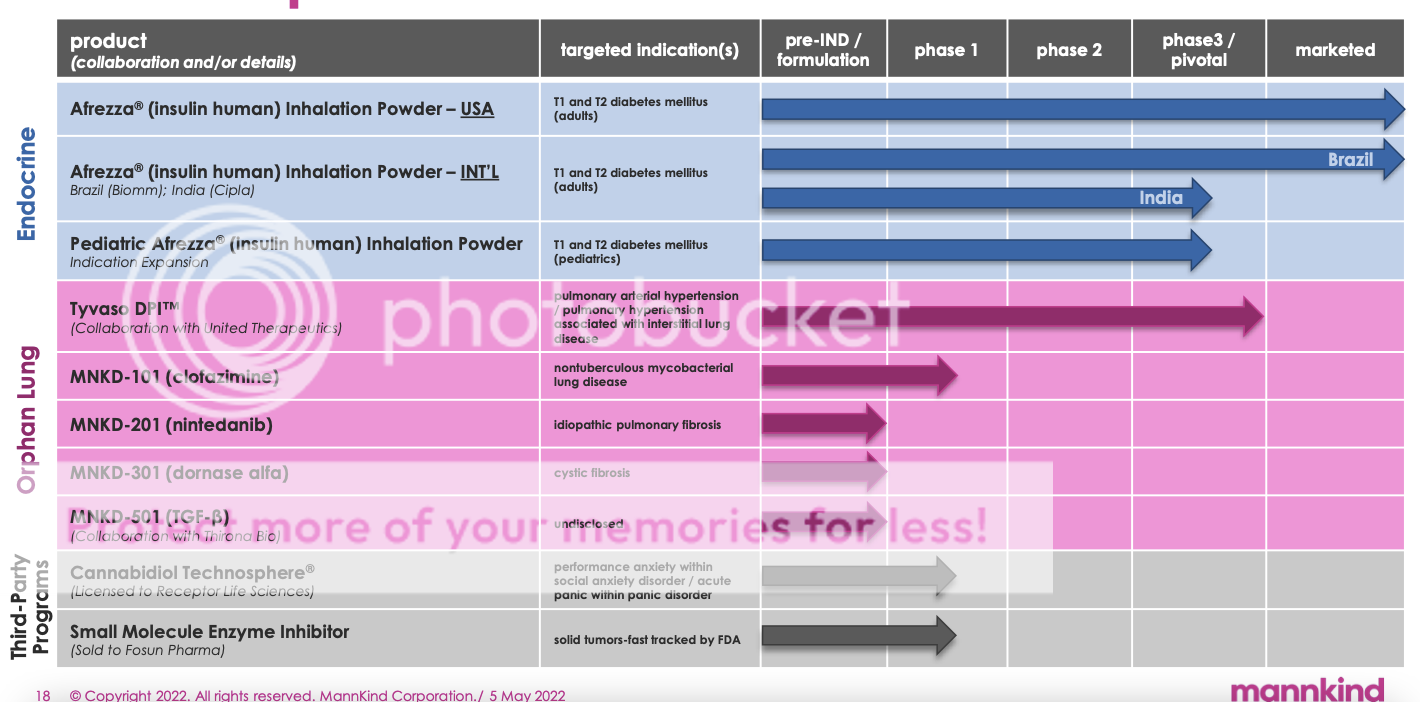 |
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Jun 1, 2022 18:55:36 GMT -5
I think the next big catalyst will be an afrezza "sales" partnership but who knows. V-Go came out of left field. Getting as many Tyveso patients on DPI is UTHR's job. Mike's job is to make Tyvaso DPI and sell afrezza. The next big catalyst will be getting Clozafimine through Phase 1 and signing up a partner which could take place by year end and then the 3 year track will start to get it to market. MNKD could buy more assets. I don't think a partnership will come for Afrezza until PEDS is about to be approved. |
|
|
|
Post by oldfishtowner on Jun 1, 2022 19:39:09 GMT -5
How about that potential Roche partnership that Castagna whispered as an aside some time ago in a presentation? I seem to recall that he mentioned it as a possibility after Tyvaso DPI approval. Is that still in play? Was it ever a real possibility?
|
|
|
|
Post by peppy on Jun 1, 2022 19:57:00 GMT -5
|
|
|
|
Post by mango on Jun 1, 2022 21:25:47 GMT -5
MannKind Catalysts
🥭 Clofazimine Phase 1 readout 3Q 2022
🥭 Clofazimine FDA IND submission 4Q 2022
🥭 Nintedanib FDA pre-IND meeting with FDA 4Q 2022
🥭 Afrezza ABC Phase 4 clinical trial complete 4Q 2022
🥭 Afrezza BluHale VIS Launch Plan ready 4Q 2022
Longer term and speculative catalysts
🥭 Second United Therapeutics collaboration pipeline candidate announcement
🥭 Receptor Life Science Technosphere Cannabidiol — it could be 3-5 more years, depending on many things. My guess is any potential partners for this will want to see FDA approved clinical trial data first. Would be nice if RLS got a partnership with Jazz Pharmaceuticals.
🥭 MannKind other pipeline candidates—apparently a new pipeline asset, indication, or approval will take place each year after 2025. That’s rather ambitious, and we have heard it before from a former CEO, but we shall see.
🥭 Partnerships for our pipeline assets
🥭 Afrezza India Phase 3 clinical trial
🥭 Afrezza Pediatrics Phase 3 clinical trial
That’s all I got right now
|
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Jun 1, 2022 22:21:45 GMT -5
How about that potential Roche partnership that Castagna whispered as an aside some time ago in a presentation? I seem to recall that he mentioned it as a possibility after Tyvaso DPI approval. Is that still in play? Was it ever a real possibility? Mike likes to throw out "BUZZ WORDS" to keep shareholders engaged. Most has been bullshit. He has mentioned EPIHALE, Roche, Novartis, MIGRANE Inhaler, etc. Most Small Cap CEOs do this crap. |
|
|
|
Post by cjm18 on Jun 1, 2022 22:58:13 GMT -5
How about that potential Roche partnership that Castagna whispered as an aside some time ago in a presentation? I seem to recall that he mentioned it as a possibility after Tyvaso DPI approval. Is that still in play? Was it ever a real possibility? Mike likes to throw out "BUZZ WORDS" to keep shareholders engaged. Most has been bullshit. He has mentioned EPIHALE, Roche, Novartis, MIGRANE Inhaler, etc. Most Small Cap CEOs do this crap. If you still hold shares it must have worked. |
|
|
|
Post by sayhey24 on Jun 2, 2022 7:16:42 GMT -5
I think the next big catalyst will be an afrezza "sales" partnership but who knows. V-Go came out of left field. Getting as many Tyveso patients on DPI is UTHR's job. Mike's job is to make Tyvaso DPI and sell afrezza. The next big catalyst will be getting Clozafimine through Phase 1 and signing up a partner which could take place by year end and then the 3 year track will start to get it to market. MNKD could buy more assets. I don't think a partnership will come for Afrezza until PEDS is about to be approved. I don't think PEDS will drive the afrezza partnership. The big market for afrezza is with the T2s. While PEDS will give it additional credibility it is a completely different market and sales approach. It is also a significantly smaller market. I think the next big catalyst will be a partnered Affinty-2 follow-up with CGMs and proper dosing narrowed to the GLP1s. If they can show superiority in that trial the sky is the limit. |
|
|
|
Post by neil36 on Jun 2, 2022 7:43:58 GMT -5
I seem to remember recently reading that both RLS and Thirona Bio (MNKD 501) are supposed to release trial data by the end of 2Q. That would be by the end of June. But now I can't find the references.
Anyone have that at their fingertips?
|
|
|
|
Post by hellodolly on Jun 2, 2022 7:50:17 GMT -5
|
|
|
|
Post by neil36 on Jun 2, 2022 8:04:53 GMT -5
Thanks Hellodolly.
Positive data from those trials will reinforce the thesis that, over time, there will be more and more revenue streams flowing to MNKD.
|
|
|
|
Post by mango on Jun 2, 2022 10:34:40 GMT -5
Most companies never make it past Phase 3, let alone an FDA approval with a clean label, and even more so with a drug-device combo (even more difficult). This is MannKind’s second time and we got the clean label we all knew we would (based on the science). Our dry powder and inhaler are completely validated. We have successfully gotten FDA approval for two drug-device products using Technosphere and our dreamboat, with two completely different API. That’s quite an achievement.
This approval puts us in a position of strength.
|
|