|
|
Post by cretin11 on Aug 10, 2022 11:17:22 GMT -5
Selling off Afrezza right before pediatric approval? There could not be worse time to sell Afrezza. You might be correct about that. On the other hand, a case could be made that it is the best time to sell it: In the hands of the right company, they could run with peds approval and do a proper job of maximizing Afrezza's incredible potential to help PWDs. Such a company would know the value and pay appropriately for it. Unfortunately it is not guaranteed that MannKind is capable of maximizing that potential. In fact, our track record clearly indicates that the opposite is more likely true. The devil is in the details. Selling Afrezza to another Sanofi that would drag their feet and/or bury it? Of course no thanks! But for the right price to MNKD and with the right approach to selling it, it would be a win-win (for us/MNKD as well as PWDs). |
|
|
|
Post by tarheelblue004 on Aug 10, 2022 11:31:40 GMT -5
I was able to listen to the call earlier today and was pretty surprised by the negative commentary from last night. I don’t think we got much new info we didn’t already know. I guess how I see it: - The bad: Shutting down PCP pilot - The good: V-Go acquisition was a slam dunk, already 40% margin, and is accretive next year - The unspoken: Guidance on how much we can expect Tyvaso DPI to increase revenue / profits over the next few quarters and years. I think the unspoken is probably what has everyone in a tizzy. Anyone who is not blind can see that Tyvaso DPI is about to transform MNKD financials. But without any specifics on when and how much, folks have nothing to point to. Mike’s hands seem to be tied by UTHR, to where we may just have to see as the revenue comes in quarter after quarter. Fine by me - I’ll be buying until Wall Street catches up  |
|
|
|
Post by biffn on Aug 10, 2022 11:54:30 GMT -5
I thought Mike’s brief but positive comments on the RLS103 CBD trial sounded very hopeful, and could evetually dwarf the rest.
|
|
|
|
Post by agedhippie on Aug 10, 2022 12:10:48 GMT -5
Having read the transcript I will correct myself on the $6M promotion that was terminated. That wasn't the "Seeing is Believing", it was the program to reach out to primary care doctors with 25 additional reps focused on helping drive success in primary care, where there is very low awareness of Afrezza adoption or trial. I guess the theory was if you drive up awareness you will get more prescriptions. However, I believe this related to managing Type 2 patients (that would fit the audience) so you will be running against the SoC with an audience who depends on the SoC for treatment protocols.
|
|
|
|
Post by agedhippie on Aug 10, 2022 12:31:31 GMT -5
Aged - you are right the statement is not quantified. From some social media sites potential patients are saying their doctor said no, afrezza is not for you. They seem to be like the "Soup Nazi". In a lot of cases new afrezza users are saying they are their doctors first afrezza patient. For the T1s "the fix" is the kids trial. If the kids trial ends up good and it seems like it is afrezza with the T1s should mimic what happened with the pumps. In the kids study they have been uping doses and "dosing as needed". For the T2s a totally different trial needs to be done. Affinity2 already showed afrezza to be superior but Sanofi wasted the moment and never followed up. As Mike said in the podcast it was done so long ago the "research community" has forgotten and has gotten busy with the GLP1s/DDP4s/SGLT2s. Mike needs to develop a plan on how to attack this market and then execute it. A new trial needs to be part of this. Clearly, whatever they did with "Seeing is Believing" was a failed mission and cost $6M. Maybe they can learn a lesson or two from this $6M failure. BTW - Mike said in the podcast they have already submitted for label changes. He did not say what changes they have requested. There is no driver to get the endos to prescribe Afrezza for their Type 1s. The positive data is largely anecdotal, or pilots/small studies and that will not move endos. My experience is that if the person is persistent enough the endo will prescribe it provided there is insurance cover. That is not going to give you a big subscriber pool though. What will need to happen off the back of the kids trial will and adult trial with the better protocol. Without that the response is going to be that it works for kids, but not for adults (cue arguments about metabolism, weight, age, diet, etc.) This will take trial data to move the needle. On the plus side it can be prescribed to kids so it expands the market regardless. Affinity-2 is a red herring. Every diabetes drug company out there has the same trial data - if you add our product when the previous step fails there is a drop in HbA1c compared to simply continuing the failed protocol. Mounjaro, to name just one, has exactly the same trial and produced a 2.39 point drop. You are perfectly right though; there needs to be a new trial where the next step is either GLP-1 or Afrezza - a good result there would be a gamechanger. |
|
|
|
Post by sayhey24 on Aug 10, 2022 13:22:35 GMT -5
Aged - you are right the statement is not quantified. From some social media sites potential patients are saying their doctor said no, afrezza is not for you. They seem to be like the "Soup Nazi". In a lot of cases new afrezza users are saying they are their doctors first afrezza patient. For the T1s "the fix" is the kids trial. If the kids trial ends up good and it seems like it is afrezza with the T1s should mimic what happened with the pumps. In the kids study they have been uping doses and "dosing as needed". For the T2s a totally different trial needs to be done. Affinity2 already showed afrezza to be superior but Sanofi wasted the moment and never followed up. As Mike said in the podcast it was done so long ago the "research community" has forgotten and has gotten busy with the GLP1s/DDP4s/SGLT2s. Mike needs to develop a plan on how to attack this market and then execute it. A new trial needs to be part of this. Clearly, whatever they did with "Seeing is Believing" was a failed mission and cost $6M. Maybe they can learn a lesson or two from this $6M failure. BTW - Mike said in the podcast they have already submitted for label changes. He did not say what changes they have requested. There is no driver to get the endos to prescribe Afrezza for their Type 1s. The positive data is largely anecdotal, or pilots/small studies and that will not move endos. My experience is that if the person is persistent enough the endo will prescribe it provided there is insurance cover. That is not going to give you a big subscriber pool though. What will need to happen off the back of the kids trial will and adult trial with the better protocol. Without that the response is going to be that it works for kids, but not for adults (cue arguments about metabolism, weight, age, diet, etc.) This will take trial data to move the needle. On the plus side it can be prescribed to kids so it expands the market regardless. Affinity-2 is a red herring. Every diabetes drug company out there has the same trial data - if you add our product when the previous step fails there is a drop in HbA1c compared to simply continuing the failed protocol. Mounjaro, to name just one, has exactly the same trial and produced a 2.39 point drop. You are perfectly right though; there needs to be a new trial where the next step is either GLP-1 or Afrezza - a good result there would be a gamechanger. I think for the T1s their is a decent game plan. We have the kids trial and the ABC trial. We also have the label changes coming but we will need to see what they are. My concern is Mike is still thinking about the T2 approach five years into the job. He is very gun shy about going head to head against the GLP1s and BP which I understand. However the T2 market is where the money is. While we can call the Affinity2 a red herring it still showed superiority and we know from clinical experience afrezza is performing better than most expected including me. Sanofi was suppose to build on that trial and position afrezza in the SOC for T2s. Now Mike either needs to partner or put on his big boy pants and go it alone. I am OK adding afrezza to Mounjaro and Jardiance and for now sucking third tit. Right now we are not even sucking hind tit. I am also OK with putting the Special Ops team together and targeting large GPs in a REVISED "Seeing is Believing" campaign. What happened in the spring that failed and cost $6M I would love to know. |
|
|
|
Post by phdedieu12 on Aug 10, 2022 13:45:48 GMT -5
And how do you KNOW that he is gun shy about going head to head against the GLP1s exactly??
|
|
|
|
Post by sayhey24 on Aug 10, 2022 14:23:20 GMT -5
And how do you KNOW that he is gun shy about going head to head against the GLP1s exactly?? Lets just say reading between the lines; Dave Kendall crashed on burned; Sanofi screwed the pouch; the spring "Seeing is Believing" crashed and burned and he lost $6M; his big booth at ADA 2022 had no live head to head demo's against Mounjaro; to name a few Mike is sitting on a paradigm shift in the treatment of T2 diabetes. Pfizer threw the kitchen sink at it and then Sanofi slow walked it. Mike is not stupid. He knows if a T2 is prescribed afrezza early most will never need the other drugs. Wouldn't you be gun shy knowing the landscape and having this history of failure and what disrupting a $40B market means? With that said there is a sweet spot with the GLP1s. These will still be prescribed for weight loss but a company like Lilly has Jardiance when the GLP1 fails. If you are taking the afrezza your sugar should not be high enough to piss out sugar and need an SGLT2. |
|
|
|
Post by peppy on Aug 10, 2022 14:23:33 GMT -5
Aged - Mike said - "the patients are asking the doctors for afrezza and the doctors won't prescribe" There is a pretty good buzz starting with the T1s on social media about afrezza. Articles like Ginger Vieina's only help. There are lots of stories about the patients being told afrezza is not for them. If they can get the afrezza things like the facebook group will help with the cough and dosing. The problem is that the " the patients are asking the doctors for afrezza and the doctors won't prescribe" statement is not quantified. What is the reason, and what are the numbers? This is a guess but I suspect the story goes; the doctors are endos and may well have tried on a couple of patients with poor results, and at that point they will stop (you don't keep throwing patients with a life threatening condition at a failing protocol). All following patients will be told that the patients they prescribed it for didn't do well so they cannot advise it. The patient's response is going to be to shrug and move on. So what is the fix? As I have been saying for ages this is going to take a large scale trial to produce the data. The trial can be with a revised protocol that increases doses, has a second dose, and is taken at the right time. Even throw in a Libre since they are freely available on insurance now. The data from that can rewrite the label and we stop getting doctors discouraged by a failing protocol. This is not rocket science, there is a reason manufactures continue large trials post launch, they want to improve the label. I think the fix should be the comparable label should be comparable and the physicians should be able to choose. I think the pharmacy purchasing managers and their rebate system need to be changed. I think comparable should be able to get health insurance coverage. Look at how many, pee your glucose out your bladder medications there are, which is superior? The one that doesn't make your leg fall off? All are covered by insurers? The problems with the trial, is 1. patient compliance with instructions. (Both subq and inhaled. Subq, you are supposed to administer 30 mins prior to eating, you do not to that. Inhaled, you are supposed to take an additional dose after 45 mins if CMG is reading say 120mg/dl. Subq people a bit nervous to do that is my guess. 2. Didn't I learn here that different people have different insulin sensitivity? A trial of people using the same protocols with different sensitivities? or multiple carbs by sensitivity and get the magic dose number? You are saying the only way to succeed is to prove superiority..... |
|
|
|
Post by sayhey24 on Aug 10, 2022 14:58:16 GMT -5
Peppy - I think what Aged is saying is you need to do the trial(s). Thats Step 1.
Proving superiority with afrezza will be a no-brainer when you juice the study with CGMs and second/third dosing. Mounjaro juiced their trials with "coaching".
Step 2 is getting afrezza its proper place in the SoC based on the trial data. That will not be easy as Dave Kendall found out.
|
|
|
|
Post by runner on Aug 10, 2022 15:33:10 GMT -5
Referencing the comment that MC made yesterday about having over 400 employees, I remember at the 2015 meeting in Danbury they said the goal was to have over 400 employees. It took a while, but they got there and I read that as good news.
|
|
|
|
Post by peppy on Aug 10, 2022 15:41:05 GMT -5
Peppy - I think what Aged is saying is you need to do the trial(s). Thats Step 1. Proving superiority with afrezza will be a no-brainer when you juice the study with CGMs and second/third dosing. Mounjaro juiced their trials with "coaching". Step 2 is getting afrezza its proper place in the SoC based on the trial data. That will not be easy as Dave Kendall found out. Say hey the last small study we did, had CGM's. I know exactly what Aged is saying Sayhey. Listen to what I am saying, I think a comparable should be covered by health insurance. I'll look, for the last study. So many variables in human behavior. Sayhey, they wouldn't second dose. Fear of insulin runs deep.   |
|
|
|
Post by agedhippie on Aug 10, 2022 17:09:39 GMT -5
Say hey the last small study we did, had CGM's. I know exactly what Aged is saying Sayhey. Listen to what I am saying, I think a comparable should be covered by health insurance. I'll look, for the last study. So many variables in human behavior. ... Those two charts you showed were from meter readings, not CGMs. They just graphed finger sticks at intervals after eating. Have they done any trials using CGMs? Also, unless you are using Regular insulin you don't typically bolus 30 minutes before you eat, you bolus when you eat which is the logic behind MDI. That's what made the whole ice cream ad so dumb if it was aimed at existing insulin users (the focus was swap to a better insulin) with the result that it was dismissed in the best case, or left people wondering what else they got wrong in the worst case (sorry, but that ad always triggers me  ) As with Afrezza you may, but don't have to, want to change the timing of your bolus depending on what you are eating (bolus late on pizzas!) |
|
|
|
Post by peppy on Aug 10, 2022 17:59:29 GMT -5
Say hey the last small study we did, had CGM's. I know exactly what Aged is saying Sayhey. Listen to what I am saying, I think a comparable should be covered by health insurance. I'll look, for the last study. So many variables in human behavior. ... They just graphed finger sticks at intervals after eating. Have they done any trials using CGMs? Also, unless you are using Regular insulin you don't typically bolus 30 minutes before you eat, you bolus when you eat which is the logic behind MDI. That's what made the whole ice cream ad so dumb if it was aimed at existing insulin users (the focus was swap to a better insulin) with the result that it was dismissed in the best case, or left people wondering what else they got wrong in the worst case (sorry, but that ad always triggers me  ) As with Afrezza you may, but don't have to, want to change the timing of your bolus depending on what you are eating (bolus late on pizzas!) Aged, those two charts, were the study results. This is the second time today you were wrong? What is your blood glucose running? I hear the type 1 is confused when running low. Aged the Subq are 30 mins prior. I swear I saw 30, now 15 mins. Afrezza is at first bite. 2.2 Subcutaneous Administration HUMALOG should be given within 15 minutes before a meal or immediately after a meal. HUMALOG given by subcutaneous injection should generally be used in regimens with an intermedi www.accessdata.fda.gov/drugsatfda_docs/label/2013/020563s115lbl.pdf Subcutaneous injection: NovoLog should generally be given immediately (within 5-10 minutes) prior to the start of a meal (2.2). www.accessdata.fda.gov/drugsatfda_docs/label/2015/020986s082lbl.pdfWe all know that onset is not Peak. Peak for the subq when given subq is two hours, 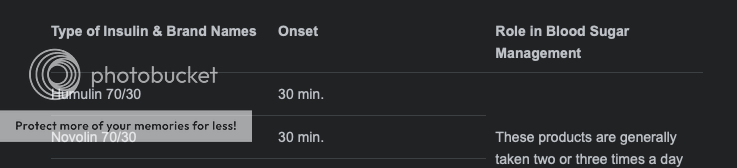 What ever you were saying about regular insulin, all changed when given IV, inhaled. Aged, I know about blousing later, VDEX and all. dosing: www.seventhform.com/vdexdownloads/vdex-whitepaper-072817.pdf page 22. Comments Afrezza’s speed of action is both a blessing and a curse. Clearly, it is a large factor in the safety of the product, but for longer meals, you may need more Afrezza to keep the post prandial levels in check. We recommend follow-on doses. For example, we advise with a standard meal to dose Afrezza 15-20 minutes after the start of the meal, and then another dose of the same size about 45 minutes later. With very long meals, we have even advised patients to administer two follow-on doses, for very tight control. Check your blood glucose level, have a small bag snack size of skittles. I listen to what you say Aged, it was skittles wasn't it. |
|
|
|
Post by sayhey24 on Aug 10, 2022 18:42:50 GMT -5
Peppy - I think what Aged is saying is you need to do the trial(s). Thats Step 1. Proving superiority with afrezza will be a no-brainer when you juice the study with CGMs and second/third dosing. Mounjaro juiced their trials with "coaching". Step 2 is getting afrezza its proper place in the SoC based on the trial data. That will not be easy as Dave Kendall found out. Say hey the last small study we did, had CGM's. I know exactly what Aged is saying Sayhey. Listen to what I am saying, I think a comparable should be covered by health insurance. I'll look, for the last study. So many variables in human behavior. Sayhey, they wouldn't second dose. Fear of insulin runs deep.   Peppy - why do you think afrezza should get comparable health insurance coverage when it does not have a comparable place in the SoC? Dave Kendall knew what needed to be done but he did not get the job done. Dave's small study you highlight above only slightly moved the needle with the T1 SoC. The kids study and the ABC study will move the needle more and at that point afrezza will demand attention for insurance coverage. The T1 plan is being executed. We just need patience with the T1s. It will follow the same model the pumps did. The HUGE problem is the T2 plan. Mike has yet to announce one and thats where the money is. When afrezza gets 10% of that market the pps is off to the moon. On the second dosing the feedback seems to be once the T1s get comfortable with afrezza correction dosing is no big deal. If you listen to the podcast I think the lady doing the interview second dosed at 120 and Mike seemed shocked. Thought leaders like Ginger Vieina in the Tudiabetes world advocated correction dosing. I would be surprised if most of the kids are not correction dosing. The problem is the newbie users who get no proper guidance. For the cough I would put a "Tip" notice on a small piece of paper in the box pointing them to a MNKD webpage providing tips on inhaling and a video. They could have another webpage with tips on dosing. |
|