|
|
Post by agedhippie on Sept 2, 2022 12:10:22 GMT -5
IMO, this is how I see things playing out the next week or so... LQDA will drift a little higher then UTHR will submit their final judgement and ask that the judge uphold the 793 as valid and infringed and then request an injunction on Yutrepia for the life of the patent. I agree. If Liquidia appeal then the stay will remain in place pending appeal, and if they don't I think UTHR will get an injunction to formalize it. Let me clarify that  ...if they don't I think then UTHR ask for an injunction to formalize it. |
|
|
|
Post by Chris-C on Sept 2, 2022 14:40:38 GMT -5
Apparently, someone forgot to tell the market that LQDA is in trouble. On the other hand, they have no issues with disposing of their mnkd shares. You have to love it- proud to be among those who enjoy water torture- MNKD style. Wake me when Mike figures out how to sell Afrezza.
|
|
|
|
Post by agedhippie on Sept 2, 2022 15:38:26 GMT -5
Do they have a manufacturing facility? I thought they are basically running the development/trials out of a "lab" facility similar to what MNKD did back in the day. If they need to develop such a facility $100m is not going to go very far. Who in their right mind is going to give this company $200m to build a manufacturing facility for a drug which is caught up in a lawsuit and could very well lose. If they win then they will need to go up against the market leader who has a drug which has a 2 year head start and an existing sales team and the drug is as good or better than theirs. I wish them luck. As far as MNKD I don't see LQDA impacting Tyvaso DPI sales for at least several years. Hopefully that will give Mike more than enough time develop a plan for afrezza and start executing it in the T2 market. If and when he does Tyvaso DPI revenue to MNKD will be insignificant. Liquidia's proprietary PRINT technology was developed and built in-house, beginning back in the days when Joe Desimone was at UNC. PRINT technology is how they make very small particles (powder) with a very tight size distribution. I think that manufacturing of all their Treprostinil powder occurs at their facilities in RTP. Their inhaler looks like they simply buy a stock item from a plastics manufacturer. Having said that, I agree that it could be tough for LQDA to hang on. It appears the only revenue stream is distributing generic Treprostinil (not in powder form) and that's not a big revenue generator. Bought to you by the boredom of a trans-continental flight and the wonders of in-flight internet  TL;DR MNKD and UTHR have this market to themselves until mid 2024 at the earliest unless the courts show an unexpected turn of speed. However, LQDA easily has enough money to last until 2025 and no expiring loan facilities until end 2025. Longer form.... Liquidia have manufacturing plant in Morrisville, North Carolina which got FDA pre-approval last year. so manufacturing plant already exists. They also have a salesforce built out already to sell the injectable version (" sales force calling on physicians and hospital pharmacies involved in the treatment of PAH"). LQDA easily has enough cash to last until launch in 2024, and their loans don't need to be refinanced until the end of 2025 (Dec 1 2025). If LQDA has to wait past 2024 they may need another raise. However, they have a facility in place giving another $13M for launch, but they are not going to hit those conditions for release now so if they want the extra cash they will need to renegotiate. They don't need the money so I think they will wait until closer to launch to renegotiate that part of the loan so they can get a better deal. It's worth bearing in mind that UTHR has an exclusive on PAH-IH until 2024 so LQDA is limited to PAH only until then regardless of any lawsuits or approvals. |
|
|
|
Post by cretin11 on Sept 2, 2022 17:06:37 GMT -5
Thanks as always for your insights, aged one.
|
|
|
|
Post by akemp3000 on Sept 2, 2022 21:25:17 GMT -5
Liquidia can now only hope and pray they actually get to launch in 2024 but even that just doesn't seem very promising at the moment...but who knows in the bizarro world of biotechs?
|
|
|
|
Post by agedhippie on Sept 2, 2022 23:55:27 GMT -5
Liquidia can now only hope and pray they actually get to launch in 2024 but even that just doesn't seem very promising at the moment...but who knows in the bizarro world of biotechs? TBH I would be pretty surprised if they don't. The PTAB have already ruled the '793 patent invalid in IPR and those reviews are not often overturned. Basically UTHR know they are going to lose but are keeping this in court to burn time. The timescale as I understand it is about a month for the response from the PTAB to respond to UTHR's filing and deny it, UTHR then has 60 days to file an appeal to Federal Court so they take the whole 60, it's then in the court's hands and they will take about a year, add a few months for the inevitable delays and you end up in early 2024. That makes the mid 2024 launch date plausible. To put numbers around UTHR's chance of success; in 2019 there were 170 appeals to Federal Court, 132 failed outright, 9 succeeded due to the an error in the IPR, and the remaining 29 succeeded owing to a legal error by the PTAB. Basically the odds of UTHR succeeding owing to an error in the IPR is around 5%. This is UTHR following the usually BP playbook of using financial muscle to shut out a competitor, which is how the game is played and what they do to Mannkind with Afrezza. In this case they are spending money in the courts rather than with PBMs and we are they beneficiaries for once. Investing in small bio-techs is a blood sport, because the big boys have the resources to block competition and are allowed to get away with it by the politicians. |
|
|
|
Post by Chris-C on Sept 3, 2022 3:11:39 GMT -5
Liquidia can now only hope and pray they actually get to launch in 2024 but even that just doesn't seem very promising at the moment...but who knows in the bizarro world of biotechs? If they do launch in 2024, they will be marketing a product to compete against one that is already available and has been prescribed by physicians for nearly two years. They had better have some great data on their advantages or they will be climbing a steep hill. MNKD knows very well the challenges of physician resistance to change. If it’s working, why take a chance on something new? The Bluhale device might offer a competitive advantage here too. |
|
|
|
Post by pat on Sept 3, 2022 6:39:37 GMT -5
july 25 at 2:43pm. “…not easy admitting LFD was right”. You’ve repeatedly written that. I don’t have the patience to pull each instance. LFD has been incredibly critical of MNKD and technosphere. I have always suspected your motives and continue to. Thanks for confirming my memory on this was accurate. You’re welcome. Just helping out a “fellow long”. |
|
|
|
Post by cretin11 on Sept 3, 2022 17:14:12 GMT -5
Thanks for confirming my memory on this was accurate. You’re welcome. Just helping out a “fellow long”. Weird to put that in quotes, it indicates sarcasm. I didn’t take you for a short but whatever works for you. You might wanna consider closing that position, we are on the verge or so I hear. |
|
|
|
Post by sportsrancho on Sept 3, 2022 17:43:15 GMT -5
We are in a bear market….the stocks that can keep their heads above water will lead the way into ( fingers crossed) a new bull market next year.
|
|
|
|
Post by pat on Sept 4, 2022 7:43:47 GMT -5
We are in a bear market….the stocks that can keep their heads above water will lead the way into ( fingers crossed) a new bull market next year. how will the repo market fare with the QE roll off? |
|
|
|
Post by caesar on Sept 4, 2022 16:52:21 GMT -5
We are in a bear market….the stocks that can keep their heads above water will lead the way into ( fingers crossed) a new bull market next year. how will the repo market fare with the QE roll off? The Fed’s antidote to repo market dysfunction is stepping in as a key transactor, with the U.S. central bank conducting both repo and reverse repo operations to keep the federal funds rate in its target range. That process is spearheaded by the New York Fed, which leads the U.S. central bank’s open market operations - welcome to the 21st century Federal Reserve System's link to the marketplace. |
|
|
|
Post by peppy on Sept 4, 2022 18:26:07 GMT -5
how will the repo market fare with the QE roll off? The Fed’s antidote to repo market dysfunction is stepping in as a key transactor, with the U.S. central bank conducting both repo and reverse repo operations to keep the federal funds rate in its target range. That process is spearheaded by the New York Fed, which leads the U.S. central bank’s open market operations - welcome to the 21st century Federal Reserve System's link to the marketplace. I have been trying to learn more about the repo market. The borrower puts up collateral, the Lender lends the money over night on the collateral. The borrower also pays the lender with a "haircut" Some collateral is hard to value. The market is 2 to 12 trillion USD in over night money. I have learned about the reverse repo also, although I can not at this moment define it. A back up to the repo market? Here is something I did not know. the March 2020 crash was a repo market crash. 10,000 to 6,631. Something about the bid, as in no bids? 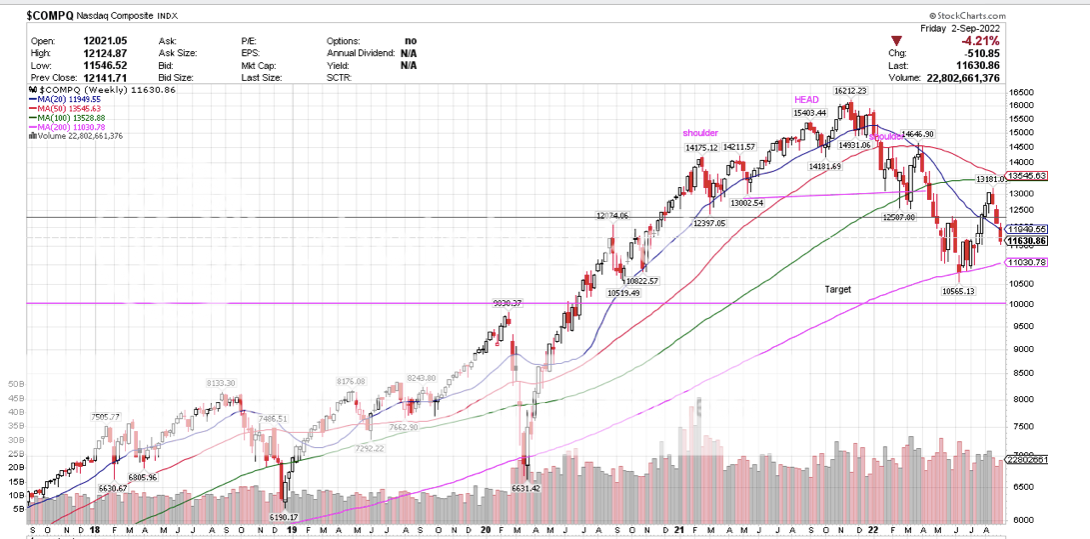  www.youtube.com/watch?v=-jxpenWInjw www.youtube.com/watch?v=-jxpenWInjw |
|
|
|
Post by neil36 on Nov 24, 2022 8:30:24 GMT -5
|
|
|
|
Post by prcgorman2 on Nov 24, 2022 9:34:51 GMT -5
Nice summary of the August judgement.
|
|