|
|
Post by matt on Jun 6, 2016 15:21:03 GMT -5
Not to put words in anyone's mouth, but capnbob appears to be using a definition that describes "Onset of Action" as the of time it takes from when the insulin is administered to when it enters the bloodstream...similar to the definition used within the Humalog website.
However, for the sake of discussion, that is not the definition defined by the authors. Regarding the abstracts under discussion, the authors published a specific definition of the term "onset of action" that is used in the study and presented in the abstract/publication.
Therefore, if you, capnbob , or anybody else wishes to unilaterally alter the authors' definition of "Onset of Action" to your own definition, you can basically say anything you want and it's supported by your specific definition(s).
Unfortunately (or fortunately, depending how you look at it) you are no longer validly arguing the merits of the authors' presentations.
Fair point, there are a lot of terms thrown around that would be more helpful if they were more precisely defined and used in a consistent way. The other point with regard to posters is that these are not super rigorous scientific studies meant to reach a supportable conclusion; they are often small studies taking a quick look at something of interest. The abstract comparing Lispro to Afrezza was done on just 30 subjects and, statistically speaking, it is hard to support any particular conclusion over another with a high degree of confidence such as what would be required to get a label. For that you need several hundred subjects stratified for age, sex, and ethnicity, and that is especially necessary in a statistically noisy diabetic population with so many comorbid conditions.
Most of all, realize that these are poster sessions and not plenary sessions or therapeutic tracks with Powerpoint presentations. Those researchers who are interested after skimming the abstract will note the number, will seek out the poster, and can grab a copy of the write-up for later consideration. Posters are meant to further research by sharing how others have designed experiments, and provide the opportunity to meet other researchers with similar interests. The posters are not seen at all by the vast majority of attendees who are too brain dead after sitting through six hours of meetings in dark rooms, sitting on bad chairs, trying to absorb an endless stream of Powerpoint slides. Those who try rapidly succumb to eyeball overload and complaining feet (for some reason poster sessions are always held on concrete floors). |
|
|
|
Post by mnholdem on Jun 6, 2016 18:42:36 GMT -5
The large number of Afrezza users studied was one of the reasons I found the lung function abstracts to be the most important. The findings of the effects of Afrezza on lung function utilizes combined data from more than 3,000 Afrezza users on the one and more than 2,000 Afrezza users on the other over a 2-year period. The presentation of those studies, one of which shows a very small decline in lung function over the first few month but no further decline for the remaining 24 months, can be assigned a higher level of credibility because of the large number of patients.
|
|
|
|
Post by brotherm1 on Jun 6, 2016 21:18:03 GMT -5
Great obsevation mnholdem about the extent of the lung tests. Makes sense they would have put more rescources into the lung studies. Castagna did indicate when answering a shareholder's question at the shareholders' meeting that the studies could lead to a label change.
|
|
|
|
Post by capnbob on Jun 6, 2016 22:31:17 GMT -5
What makes this discussion frustrating is that there are so many different definitions of the term "onset of action" that, depending on the definition of the person posting, EVERYONE could, by their own definitions, be correct.
Here are only a few of the definitions published by prominent medical resources:
Onset of Action
- "The time required after administration of a drug for a response to be observed." - Mosby's Medical Dictionary, 9th edition. © 2009, Elsevier.
- "Pharmacology: The length of time needed for a medicine to become effective." - McGraw-Hill Concise Dictionary of Modern Medicine. © 2002 by The McGraw-Hill Companies, Inc.
- "The time from drug administration until the drug exerts an observable specific effect or response." - Medical Dictionary for the Health Professions and Nursing © Farlex 2012
- "The time between the administration of a medication or other form of treatment and the first evidence of its effect." - Medical Dictionary, © 2009 Farlex and Partners
- "The time it takes a drug to reach the minimum effective concentration after a drug is administered." - Key Terms - Pharmacology: A Nursing Process Approach (6th ed)
- "Onset of action is the duration of time it takes for a drug's effects to come to prominence upon administration." - Wikipedia
Naturally, a debater making an argument will choose the definition that best suits his/her argument.
---
Not to put words in anyone's mouth, but capnbob appears to be using a definition that describes "Onset of Action" as the of time it takes from when the insulin is administered to when it enters the bloodstream...similar to the definition used within the Humalog website.
However, for the sake of discussion, that is not the definition defined by the authors. Regarding the abstracts under discussion, the authors published a specific definition of the term "onset of action" that is used in the study and presented in the abstract/publication.
Therefore, if you, capnbob , or anybody else wishes to unilaterally alter the authors' definition of "Onset of Action" to your own definition, you can basically say anything you want and it's supported by your specific definition(s).
Unfortunately (or fortunately, depending how you look at it) you are no longer validly arguing the merits of the authors' presentations.
This is one reason why such data should be presented as a graph. In the graph from the insert, I define the "onset" to be the zero point where you can see the actual effect of the insulin on glucose kicking in. Referring to the insert graph, from "0" to 50% max is about 15-20 minutes using my definition starting at "0." That makes sense to me and correlates with what is presented in the abstract. If the abstract authors were using your definition, then why not just present the actual "onset of action" time? Why confuse the viewer by presenting onset of action to 50% GIR? Or at least present the actual "onset of action" time in addition to the rest. Also note that if you take the difference between the abstract's afrezza time and the lispro time to 50%GIR for comparable doses, it is about 30 minutes. That fits what the insert graph says as well. But, by your definition, there should have been a 25-25 minute larger gap since, in effect, the lispro graph is being shifted to the right by its longer time of onset. Regardless, a simple graph would have been a whole lot better way to present the data. |
|
|
|
Post by capnbob on Jun 6, 2016 22:42:01 GMT -5
The large number of Afrezza users studied was one of the reasons I found the lung function abstracts to be the most important. The findings of the effects of Afrezza on lung function utilizes combined data from more than 3,000 Afrezza users on the one and more than 2,000 Afrezza users on the other over a 2-year period. The presentation of those studies, one of which shows a very small decline in lung function over the first few month but no further decline for the remaining 24 months, can be assigned a higher level of credibility because of the large number of patients. Is the new data significantly different from the old data:  |
|
|
|
Post by brotherm1 on Jun 6, 2016 23:08:05 GMT -5
Question for the moderators: Is there a way you can at least take a couple of stars away from a poster?
|
|
|
|
Post by tchalaa on Jun 7, 2016 3:06:04 GMT -5
Several hundred subjects stratified for age, sex, and ethnicity participated to this study: Evaluate Safety of Technosphere® Insulin (TI) in Diabetic Subjects With Moderate Obstructive Pulmonary Disease
Especially necessary in a statistically noisy diabetic population, a total of 517 participants were screened in 5 countries . First participant was screened in March 2009. Source: clinicaltrials.gov/ct2/show/results/NCT00642616?term=Technosphere%C2%AE+Insulin&rank=4 |
|
|
|
Post by LosingMyBullishness on Jun 7, 2016 5:16:28 GMT -5
Please help me in the evalutation 937-P / 937 - Effects of Inhaled Technosphere Insulin (TI) on the Pulmonary Function of Patients with T1D and T2D:
Before you apply your flamethrowers please bear in mind that I am long-term shareholder with far to much in MNKD for common sense.
When I first looked at it I was frustrated as I saw a statistically impact of TS Insulin on FEV. I doubted that in the past as I could not unterstand why TS should have a decremental effect on FEV as TS quickly dissolves in the lung and the portion of insulin is tiny.
- Do you have an explanation for the decline with TS? I understand it is reversable. I assumed that the spiro test was executed not directly after applying Afrezza.
- What does it mean: a very small percentage: Let's go with 1-2 % of FEV. Is this small? The comparator got to that decline after 18 month. So using TS made the patent lose 18 month of standard lung function decline.
- When I look at the slope of both lines I see a slightly steeper decline with TS. The FEV seems to decrease more or less steady over life. If the slope with TS is only slighter steeper it could have a significant impact over time.
All medications have sideeffects. My fear is that Endos see the difference in the graph against the standard forgetting that the alternative has sideeffects that Afrezza has not. And do Endos (who I assume are less knowledgable about lung function) share the (paid) perception of the authors that this is small?
If I walked by I would catch that there is a difference in the curves proving that Afrezza really has a negative impact on the lung function. I might start a discussion with the presenter but my mindset would already be: Okay, that is a real issue and sure, that MNKD guy is now trying to convince me that it is not important.
Has someone a FEV graph comparing smokers and non-smokers? That would help to put the small decline in perspective.
|
|
|
|
Post by slapshot on Jun 7, 2016 6:29:14 GMT -5
Please help me in the evalutation 937-P / 937 - Effects of Inhaled Technosphere Insulin (TI) on the Pulmonary Function of Patients with T1D and T2D: Before you apply your flamethrowers please bear in mind that I am long-term shareholder with far to much in MNKD for common sense. When I first looked at it I was frustrated as I saw a statistically impact of TS Insulin on FEV. I doubted that in the past as I could not unterstand why TS should have a decremental effect on FEV as TS quickly dissolves in the lung and the portion of insulin is tiny. - Do you have an explanation for the decline with TS? I understand it is reversable. I assumed that the spiro test was executed not directly after applying Afrezza. - What does it mean: a very small percentage: Let's go with 1-2 % of FEV. Is this small? The comparator got to that decline after 18 month. So using TS made the patent lose 18 month of standard lung function decline. - When I look at the slope of both lines I see a slightly steeper decline with TS. The FEV seems to decrease more or less steady over life. If the slope with TS is only slighter steeper it could have a significant impact over time. All medications have sideeffects. My fear is that Endos see the difference in the graph against the standard forgetting that the alternative has sideeffects that Afrezza has not. And do Endos (who I assume are less knowledgable about lung function) share the (paid) perception of the authors that this is small? If I walked by I would catch that there is a difference in the curves proving that Afrezza really has a negative impact on the lung function. I might start a discussion with the presenter but my mindset would already be: Okay, that is a real issue and sure, that MNKD guy is now trying to convince me that it is not important. Has someone a FEV graph comparing smokers and non-smokers? That would help to put the small decline in perspective. I believe i see what you mean (assuming its the graph a couple of posts above), it would have been good to include a third curve (or another graph) representing the difference, which seemingly would increase for the first 3 months then basically flat line. Another alternative would be a curve that represents the acceleration of the difference which would only occur over the first 3 months and then be about 0. |
|
|
|
Post by cm5 on Jun 7, 2016 7:05:39 GMT -5
|
|
|
|
Post by mnholdem on Jun 7, 2016 7:16:33 GMT -5
The large number of Afrezza users studied was one of the reasons I found the lung function abstracts to be the most important. The findings of the effects of Afrezza on lung function utilizes combined data from more than 3,000 Afrezza users on the one and more than 2,000 Afrezza users on the other over a 2-year period. The presentation of those studies, one of which shows a very small decline in lung function over the first few month but no further decline for the remaining 24 months, can be assigned a higher level of credibility because of the large number of patients. Is the new data significantly different from the old data:  The new abstracts do not reveal anything NEW, if that's what your question implies. The old graph (above) could actually be call the "current" graph, as it is the graph displayed in Afrezza's label as currently approved by the FDA. But the new abstracts are significant, not so much in new results, but rather in the size of the number of patients measured. The new graphs, in the eyes of attending physicians, will be considered to be more reliable and to have more credibility, even if the data itself simply confirms the previous findings.
--- From new abstract:
"Spirometry results were assessed using pooled analyses of 7 TI studies (duration 6-24 months). We included 1,842 patients with T1D (mean age 39 years; 51% male) and 2,281 with T2D (mean age 56 years; 56% male) using TI or comparator (RAI, standard of care, placebo).
Baseline mean (SD) forced expiratory volume in 1 second (FEV1) values were 3.49 (0.787) L and 3.01 (0.714) L for patients with T1D and T2D, respectively. FEV1 in both TI and comparator groups declined from baseline to 3 months in patients with T1D (TI = -0.063 L, comparator = -0.019 L; P = 0.1414) and T2D (TI = -0.084 L, comparator = -0.043 L; P = 0.1431) (Figure). After 3 months the decline in FEV1 at each time point was comparable between TI and comparator groups. These reductions were a small percentage of the FEV1, and not influenced by significant outliers. In 2 studies where FEV1 was measured after discontinuation, there was no difference between groups after 1 month.
Our data, pooled from 7 clinical trials, demonstrate that TI’s effects on pulmonary function are small and develop during the first 3 months of use. After that, TI and comparator groups demonstrate comparable physiologic declines for up to 24 months."
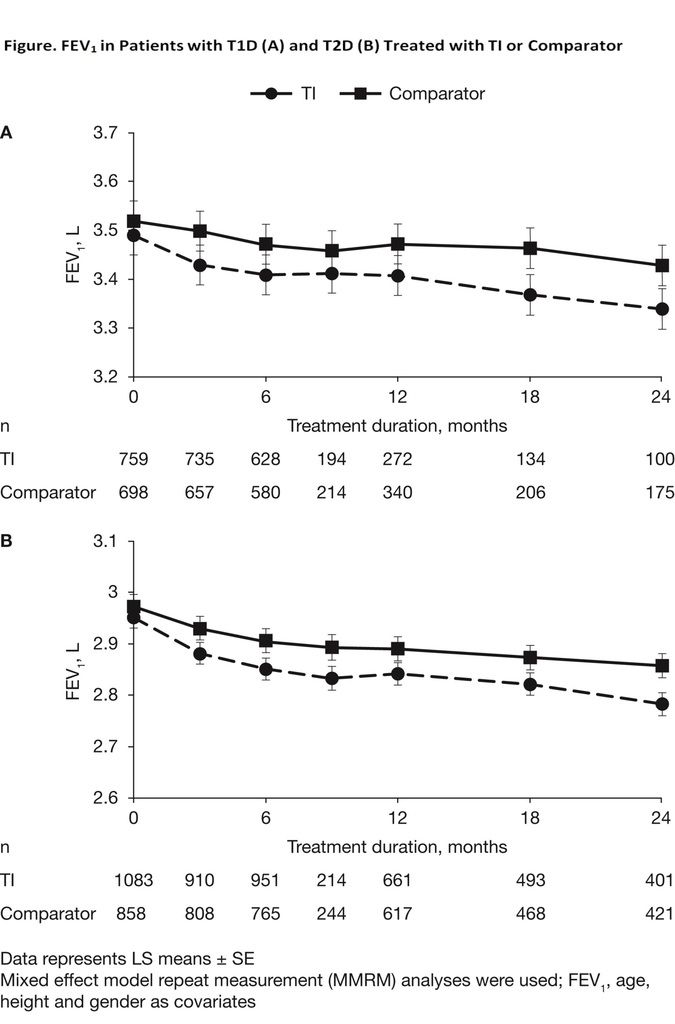
---
Of note is the difference in FEV1 volume between Graphs A (Type 1 Diabetes) & Graph B (Type 2 Diabetes). The lower FEV1 (forced expiratory volume in 1 second) of patients with Type 2 diabetes is noted in the other pulmonary function abstract and is explained by the fact that patients in these studies who had Type 2 diabetes constituted an older group within the study (which perhaps mirrors our society as a whole). Declining lung function is a normal occurrence with age, which may explain why even the comparator (non-TI) FEV1 lines on the graphs show a decline over time. Although the older T2 group starts with lower FEV1 numbers than the younger T1 group, the graphs demonstrate that the use of Technosphere Insulin (TI) causes a slight decline (.0248 and .0442 respectively) in FEV1 over the first three months and afterward does not cause additional deterioration of lung function on top of the decline that is already naturally occurring through time. So it would appear that the newer data combining 7 studies confirms that the decline in lung function, which is naturally caused due to aging, is not likely to be further accelerated by Technosphere Insulin.
|
|
|
|
Post by cm5 on Jun 7, 2016 7:20:17 GMT -5
And, a view above the forest re: duration-----
|
|
|
|
Post by cm5 on Jun 7, 2016 7:44:34 GMT -5
And, given the discussion about analogues--
Looking at the carcinogenicity of human insulin analogues via the intrinsic disorder prism
|
|
|
|
Post by bradleysbest on Jun 8, 2016 10:33:27 GMT -5
Will anyone from this board attend the ADA? A first hand account of the weekend would be nice. Are they allowed to provide Afrezza samples to attendees this weekend?
|
|


