|
|
Post by mnkdnut on Aug 1, 2016 17:25:36 GMT -5
You may be absolutely correct, akemp, and most on this board are betting on it. However, the scientific proof on Afrezza superiority is missing (anecdotal "proof" - ok, we got lots of that). Those that hang their careers on making decisions regarding pharmaceuticals (eg. payers and prescribers) need controlled clinical studies to back up claims of actual superior outcomes. They can't afford to chase after every new shiny invention that "might" be better. The PK/PD profile data has been around since FDA approval and is a teaser of interesting potential, not a substitute for actual outcomes. For whatever reasons, MNKD's scientific and clinical staff has yet to be able to deliver scientific/clinical proof of superiority (an epic fail in my opinion. Was Al too lenient with his staff?), so the sales team must fight with one arm tied behind their backs. Sanofi should have known to address this early on, but of course we know now it was game over as soon as Brandicourt entered. I have high hopes for the pediatric study- even though it likely won't address adults as far as FDA is concerned - but we're counting on Mike C and RLS to keep the doors open until then. Heck, I'd be thrilled to see even a time-in-range study at this point. The pharmaceutical industry competes on clinical data that supports cost/benefit analyses. We can't keep hoping to win through compelling individual case histories, no matter how emotionally uplifting they are. Same old chit, different day. The reason your story does not work is we are not blind, we can see. screencast.com/t/s6neMncEshf screencast.com/t/4W2uaL19IqtW screencast.com/t/o52jfDnnZa screencast.com/t/z2NNkTxeg screencast.com/t/1lw4RXiEpU screencast.com/t/Ab2vH63GqdV screencast.com/t/buvxOYfOb screencast.com/t/A4BkzUlvr9 screencast.com/t/rU8FNwJ8o1E screencast.com/t/9M4hRMCZpp screencast.com/t/fxfLxL0g screencast.com/t/WRntikEkOpV
Same old screencasts of anecdotal information, Peppy. That's nice evidence, but not proof! No pharmaceutical company is exempt from their duty to provide scientific proof. IF they can't, you have to ask why. I prefer to be a critical investor rather than a always-forgiving fan of the company, that's all. You are welcomed to your blind optimism, but I don't have time for repetitive screencasts that don't add anything new.
|
|
|
|
Post by audiomr on Aug 1, 2016 17:32:09 GMT -5
What are you referring to as prandial drugs? Afrezza is a prandial med, so it would not be combined with another. I interpreted akemp3000 to mean other prandials in combination with Tresiba are not being reported to be performing as well as Afrezza with Tresiba. I don't think Novo-Nordisk is happy about it and I don't expect they will publicly advocate how well Afrezza works when combined with their basal Tresiba. After all, Afrezza directly competes with Novo's prandial RAA insulin Novolog.  Thanks. |
|
|
|
Post by bioexec25 on Aug 1, 2016 17:52:13 GMT -5
"I don't think Novo-Nordisk is happy about it and I don't expect they will publicly advocate how well Afrezza works when combined with their basal Tresiba. After all, Afrezza directly competes with Novo's prandial RAA insulin".
Mnholdem, interesting conundrum. Novolog making predictable cash, but it seems given the efficacy of Tresiba and Aftezza there must be thoughts by Novo & Mnkd to make it an "and" not an "or".
Peppy, suppose that falls more into the sleeping with enemies than best friends. ;-))
|
|
|
|
Post by audiomr on Aug 1, 2016 17:57:07 GMT -5
"I don't think Novo-Nordisk is happy about it and I don't expect they will publicly advocate how well Afrezza works when combined with their basal Tresiba. After all, Afrezza directly competes with Novo's prandial RAA insulin". Mnholdem, interesting conundrum. Novolog making predictable cash, but it seems given the efficacy of Tresiba and Aftezza there must be thoughts by Novo & Mnkd to make it an "and" not an "or". Peppy, suppose that falls more into the sleeping with enemies than best friends. ;-)) For what it's worth, I think Novolog goes off-patent soon. |
|
|
|
Post by dcassidy1618a on Aug 1, 2016 22:44:23 GMT -5
Exactly! Afrezza can be compared to other prandial solutions just like basal solutions can be compared with one another. The point is that this type of head-to-head comparison should now be considered antiquated or inadequate now that it "appears" the combination of Tresiba, Afrezza and a cgm might just offer the best "package" solution that diabetics have seen to date. Future comparisons should include the combination basal and prandial package proposed with "time-in-range" results from cgms. Why does MNKD never say anything along these lines? |
|
|
|
Post by liane on Aug 2, 2016 4:41:28 GMT -5
MNKD cannot. These are anecdotal findings and they are not on the the FDA label. There has to be a clinical study and then the FDA's blessing before they can say anything along these lines.
|
|
|
|
Post by mnholdem on Aug 2, 2016 7:10:03 GMT -5
MNKD cannot. These are anecdotal findings and they are not on the the FDA label. There has to be a clinical study and then the FDA's blessing before they can say anything along these lines. Until then, the only avenues available to communicate anecdotal evidence are more publications of abstracts, scientific studies, journal articles and, of course, word of mouth via blogs and social media which reach out to patients with diabetes. |
|
|
|
Post by cm5 on Aug 2, 2016 7:28:59 GMT -5
Yes, but----as per prior posts in this thread: Mannkind: A Growing Threat To The Status Quo
|
|
|
|
Post by akemp3000 on Aug 2, 2016 7:47:47 GMT -5
Anecdotal stories, social media, rumors, etc. germinate ideas that can be evaluated over time. Some can one day become proven scientific fact. It's great that we now have message boards to share and accelerate these ideas. We even get to speculate on what might be coming next. IF it turns out to be a fact that the combination of Tresiba, Afrezza and a cgm work better than any other solution available today to get the B/G of a diabetic to an increased time-in-range of a non-diabetic, then we are at a moment in time that will one day be looked back on as historic for global healthcare. This is a possibility that is great to imagine and we would have Al Mann, and others to thank. Now let's see how this evolves. Best of luck to Mike C.
|
|
|
|
Post by mnholdem on Aug 2, 2016 8:24:19 GMT -5
One thing is clear:
Companies like Dexcom are pushing the FDA to elevate the status of CGM in diabetes treatment in order to achieve better reimbursement for patients with Type I and Type II diabetes who simply cannot afford the device.
Until a CGM becomes affordable out-of-pocket or they get fully reimbursed, patients who cannot afford their own CGM may still be powerfully swayed by images published on social media which show CMG screenshots of BS control using Afrezza + basal.
We'll be seeing more of these CGM images in articles, too, such as the piece published this week by Trent Welsh in Seeking Alpha:
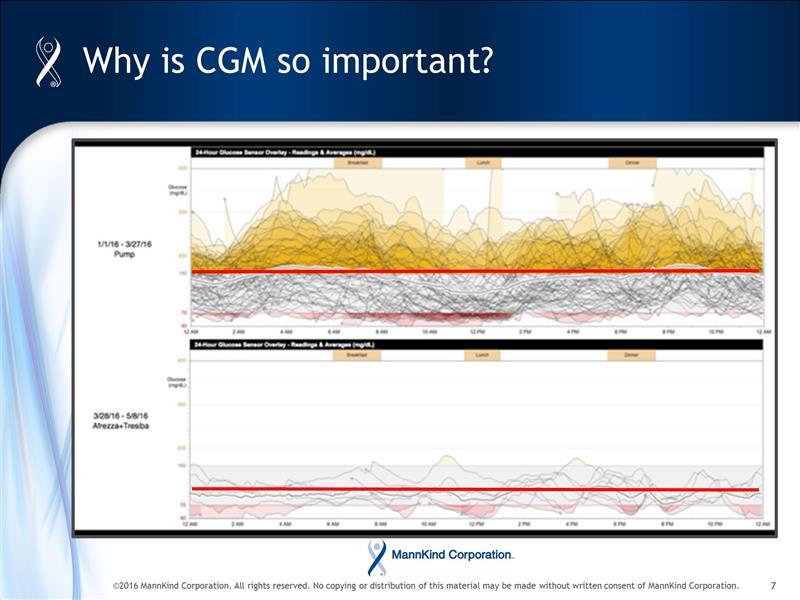
The old adage, "a picture is worth a thousand words" should not be underestimated and advocates (and publishers) who post Afrezza results using CGM images, may eventually create more awareness among patients with diabetes than some think.
|
|
|
|
Post by peppy on Aug 2, 2016 9:11:18 GMT -5
|
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Aug 2, 2016 9:20:45 GMT -5
In Castagnas last presentation he indicated on one of the slides that Afrezza's ability to control PPG results in fewer incidences of hypoglycemia and basal is not as critical for this specific aspect of control. Measuring A1C is going to go the way of the buggy whip. A1C does not speak to the rapid swings in blood glucose levels which is the culprit behind the long term health complications of people with diabetes as it is merely an average. Look up diabetes complications and control trials for additional info.
Dexcom is building another factory in Arizona. They are also working feverishly on a next generation sensor that will drive the cost down well below where it is today and it will be sold at drugstores and covered as an Rx item. Their current sensor, the G5 will send data directly to a patients smartphone. The data on the smartphone can get automatically pushed into some type of cloud database and given Dexcom's relationship with Google, it is not if but when. Once this happens, there will be limited if any need for studies to validate which Rx products or combos perform best - this data will be mined and it will become painfully obvious as to which forms of treatment reduce large swings in blood glucose levels and ultimately long term health complications. If I am a big payor, once I have this data, there will be minimal ambiguity as to which therapies are most effective.
None of the above happens in 2016. By Q2 of '17, Google should start to see larger amounts of G5 data roll into their cloud.
For now, MNKD needs strong NRx growth (18%_ W/W with a few gap ups along the way) and TRx growth to follow. This and another $60 - $80mm in cash and we should be OK.
|
|
|
|
Post by therealisaching on Aug 2, 2016 9:24:33 GMT -5
MNKD cannot. These are anecdotal findings and they are not on the the FDA label. There has to be a clinical study and then the FDA's blessing before they can say anything along these lines. Until then, the only avenues available to communicate anecdotal evidence are more publications of abstracts, scientific studies, journal articles and, of course, word of mouth via blogs and social media which reach out to patients with diabetes. From the q1 earnings call:
Additionally, our medical organization will implement clinical-based work to support the appropriate use of Afrezza in our target population.
Specifically, we tend to initiate small, fast and inexpensive studies to demonstrate improved dosing and titration recommendations for Afrezza
users. These studies will draw on the experiences of our most successful patients and doctor prescribers, and we thank them for their invaluable
inputs. We're also planning a time in range study for patients with access to continuous glucose monitors. Our plan is to target these studies for
publication initially, with a potential to evolve into a label expansion study thereafter.
|
|
|
|
Post by cm5 on Aug 2, 2016 9:56:56 GMT -5
|
|
|
|
Post by cm5 on Aug 2, 2016 10:26:05 GMT -5
Per trondisc post: "Just read the entire article assessment over at MassDevice.c0m (if I post the link here this thread will be moved over and transferred to the articles, blog, media, etc. page so just do a damn search to find it). This information caught my attention... ' With all of the data provided for the G5 CGM, Dexcom is likely to win a favorable vote in the panel, according to Leerink Partners analyst Danielle Antallfy.
In our view, the panel docs are largely benign and support our belief that the panel will vote favorably that the benefits of a nonadjunctive claim outweigh the risks,” Antalffy wrote in a letter to investors. “As a reminder, the importance of a dosing claim centers not only around increased convenience to the patient — potentially further accelerating patient adoption — and validation of the technology, which could drive a higher number of physicians to prescribe, but also the possibility of securing CMS coverage, which would provide access to the 20%+ of Type 1 patients currently covered by Medicare.”
The roster of authorities sitting on the panel will work in Dexcom’s favor as well, Antallfy said, as it includes 6 endocrinologists and diabetes authorities, who the analyst thinks would “likely be more postively biased towards CGM.' "Read more: h ttp://mnkd.proboards.com/thread/5883/dxcm-verge-approval-implications-mnkd#ixzz4GBlfZtX1
Danielle Antalffy, Wall Street Analyst at Leerink Swann
Main Sector:Healthcare
Ranked #140 out of 4,083 Analysts(#221 of 9,427 overall experts) (over one year) |
|




