|
|
Post by lakon on Jan 22, 2017 12:20:14 GMT -5
I think that dosing at the beginning of the meal is part of the problem. They need to get that changed in the label. They need to gather statistics to show that results can improve with a simple protocol, like dose 15 minutes after beginning a meal AND an hour later IF you had a large or slow to digest meal. Also, don't be afraid of larger doses when eating. Al made this point before. Let the probability be on your side. You don't need perfect timing to have much improved results. Sub-optimal dosing should still yield better outcomes than previous treatments due to more timely action near when needed, but too soon or too late is not helpful. In the moment seems best. They need studies to show this effect more clearly. Theoretically, it should work as Al said. Simple as that. After building confidence in the method, finger pricks can be rare because it just works. Of course, the extreme users with CGM can dose in real-time, and that's awesome for those who want to put forth the extra effort. For the vast majority (Type 2's), like agedhippie said, compliance is the issue so follow the KISS principle. |
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on Jan 22, 2017 20:26:48 GMT -5
Wow, it takes all kinds, stay strong. Anything to help get the word out, you would think people would be thanking you. Nope, very sad. sayhey they say he is frag on YMB. I don't know... but after he said it I posted this:-)) IMO Wild pig is not to sharp. As annoying as Fraggy is, he is not stupid |
|
|
|
Post by dreamboatcruise on Jan 23, 2017 13:47:35 GMT -5
I think that dosing at the beginning of the meal is part of the problem. They need to get that changed in the label. They need to gather statistics to show that results can improve with a simple protocol, like dose 15 minutes after beginning a meal AND an hour later IF you had a large or slow to digest meal. Also, don't be afraid of larger doses when eating. Al made this point before. Let the probability be on your side. You don't need perfect timing to have much improved results. Sub-optimal dosing should still yield better outcomes than previous treatments due to more timely action near when needed, but too soon or too late is not helpful. In the moment seems best. They need studies to show this effect more clearly. Theoretically, it should work as Al said. Simple as that. After building confidence in the method, finger pricks can be rare because it just works. Of course, the extreme users with CGM can dose in real-time, and that's awesome for those who want to put forth the extra effort. For the vast majority (Type 2's), like agedhippie said, compliance is the issue so follow the KISS principle. The simulation study that was published had followup dosing but I believe based on BG reading, which in real life would require finger stick for those not on CGM. I wonder if there would be anyway to use those same simulations to investigate the protocol you suggest... based on estimate of carb/fat content of the meal to determine need for additional post meal dosing. It would need to account for wide variances in knowledge of actual food content... but I have a hunch that what you say could well be true that getting the timing right is the crucial thing and it would still yield good results even if the guesses on food content are significantly off. |
|
|
|
Post by peppy on Jan 23, 2017 14:09:48 GMT -5
I have previously mentioned the importance of dosage timing when taking Afrezza, but have not gone into much detail. So I thought it would be worthwhile talking about what I have learned so far about timing.
As a general rule, I dose about 10 minutes after I start eating, which is before my glucose levels start to rise from the meal. As I mention in the video, the best time to dose seems to depend on the fat content of the meal. And for some high fat meals, a follow up dose of Afrezza is neccesary.
I have found the same rule also applies if a follow-up dose is required. It is important to have the follow-up dose before the levels begin to rise out of range. If I had a CGM with alerts, I would use this to notify me as soon as it levels began to increase. That way I wouldn’t miss the optimum time for the follow-up.
afrezzadownunder.com/

www.screencast.com/t/qHsWcjqc
|
|
|
|
Post by dreamboatcruise on Jan 23, 2017 14:34:38 GMT -5
Seems like the issue is getting a protocol packaged in such a way that it can be taken to doctors. I gather that there is some grey area with regard to what a sales rep can present to a doctor. I'm pretty sure that an article published in reviewed medical journal can be given to docs. I suspect showing docs anecdotal accounts from individual patients is something that can't/wouldn't be done.
I wonder if the reps currently being deployed are presenting docs with the published simulation results showing benefits of delayed/split dosing.
|
|
|
|
Post by mnholdem on Jan 23, 2017 15:10:48 GMT -5
I think that current regulations require FDA approval of any studies or reseach that can be included within the packet of marketing material. I'm wondering whether MannKind will get shorter-term approval to use the most recent study PK/PD graphics in the current label, while they wait for the longer-term approval of their submittal to upgrade Afrezza to a First-in-Class drug classification.
Matt announced that MannKind is planning studies for "starting and titrating the product quickly and effectively", thereby providing data that can be disseminated to physicians once approved by the FDA. This type of approval typical doesn't take very long.
Here is the excerpt from 3Q16 Earnings Call:
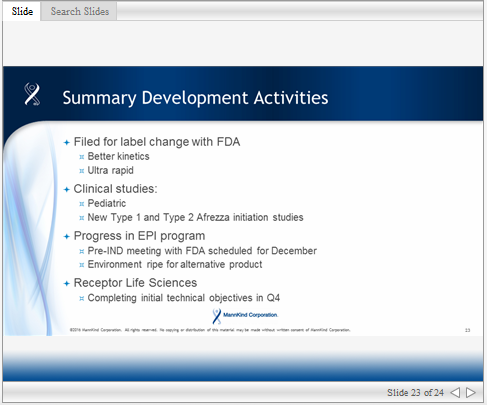
"...on the development side, we have filed in our discussions with FDA regarding an improvement to the Afrezza label, we will keep you informed as this develops. At the same time, we are investing in our future clinically not only by starting our pediatric clinical studies in Q1, but also additional studies to better demonstrate the advantages of Afrezza, Afrezza’s PK and PD profile by focusing our near-term investment in the starting and titrating the product quickly and effectively with CGM and/or standardized dose titration schedules." - CEO Matthew Pfeffer |
|