|
|
Post by Chris-C on Oct 21, 2021 11:25:37 GMT -5
I doubt any future companies will be giving money to the fda for early approval. The FDA didn't get any money for the expedited review. They dole out the vouchers for new drugs with perceived urgency. What the biotech co does with it is their business. Sometimes they sell them to other companies for big money. The FDA honors them, but I'm sure they feel that it's a misused system in cases where the review is not for the original purpose for which they issued it. goyocafe I could not agree more. It is accurate that FDA does not sell expedited review vouchers. I'm not sure who dreamed up the voucher system but it does not serve the interest of the public to have expedited reviews given for one reason and used for another while at the same time creating a back market for using the vouchers as currency. For an agency that has answered to Congress for its long review times, especially for life-saving drugs, this seems counterproductive. And clearly, the public does not understand it either. How are these valued in the eyes of analysts? If MNKD held a voucher, for example, could they claim it had value on their balance sheet knowing it could be sold for $100M? IMO, It should be a "use it or lose it within a specified timeframe for the purposes granted" type of policy. Period. Full stop. After all, where does public interest and public health come into play? Think mission, FDA lads and lasses, think mission, because the mission is why you exist and why our tax dollars fund your operations. JMHO Chris |
|
|
|
Post by mnkdfann on Oct 21, 2021 12:20:45 GMT -5
The FDA didn't get any money for the expedited review. They dole out the vouchers for new drugs with perceived urgency. What the biotech co does with it is their business. Sometimes they sell them to other companies for big money. The FDA honors them, but I'm sure they feel that it's a misused system in cases where the review is not for the original purpose for which they issued it. goyocafe I could not agree more. It is accurate that FDA does not sell expedited review vouchers. I'm not sure who dreamed up the voucher system but it does not serve the interest of the public to have expedited reviews given for one reason and used for another while at the same time creating a back market for using the vouchers as currency. For an agency that has answered to Congress for its long review times, especially for life-saving drugs, this seems counterproductive. And clearly, the public does not understand it either. How are these valued in the eyes of analysts? If MNKD held a voucher, for example, could they claim it had value on their balance sheet knowing it could be sold for $100M? IMO, It should be a "use it or lose it within a specified timeframe for the purposes granted" type of policy. Period. Full stop. After all, where does public interest and public health come into play? Think mission, FDA lads and lasses, think mission, because the mission is why you exist and why our tax dollars fund your operations. JMHO Chris I thought the expedited review vouchers were given to companies to spur investigation and development of needed drugs. What a company ultimately did with the voucher was left to the company, but either way the desired needed drug was put into development. There are a lot of subtleties to the program, but the original intent was always to allow companies to sell the vouchers if that helped them more. Note, originally a company even had to announce its intention to use a priority review voucher 365 days in advance, i.e. before they had even run trials to know if the drug was successful or not. I'm not arguing for or against anything, just pointing out there are a lot of subtleties and I think it all rather complex. This seems a reasonable 'summary' ... www.raps.org/regulatory-focus/news-articles/2017/12/regulatory-explainer-everything-you-need-to-know-about-fdas-priority-review-vouchers |
|
|
|
Post by prcgorman2 on Oct 22, 2021 9:35:07 GMT -5
I doubt any future companies will be giving money to the fda for early approval. The FDA didn't get any money for the expedited review. They dole out the vouchers for new drugs with perceived urgency. What the biotech co does with it is their business. Sometimes they sell them to other companies for big money. The FDA honors them, but I'm sure they feel that it's a misused system in cases where the review is not for the original purpose for which they issued it. So you’re saying federal employees would not respect the process that Congress made them follow (for the ostensible benefit of people who need drugs faster which is maybe all of us)? Heresy! |
|
|
|
Post by yossarian on Oct 22, 2021 13:19:45 GMT -5
|
|
|
|
Post by prcgorman2 on Oct 22, 2021 22:11:09 GMT -5
Thanks for repeating the news from 5 days ago?
|
|
|
|
Post by golfeveryday on Oct 23, 2021 6:47:05 GMT -5
If LQDA gets approved November 7 you have to factor in the revenue loss on our end. They are way up pre-market. yes, you have to factor in a potential LQDA approval. They can’t launch yet but an approval is another negative in MNKD’s favor at this point. You also have to factor in that MNKD and UTHR have not confirmed what is required to fix this CRL and what the review period will be. Many people are saying Tyvaso is essentially approved, based on what they saw from the label. The label was amazing, but UTHR got a CRL and the FDA is an uncertain entity to say the least. It is very odd to me they received a full CRL for an issue with an analytical testing facility at a third party when trepostinil is currently on the market. This is likely the main reason I sold until I hear more from UTHR on the plan. Sounds like there is more to it and I was not risking already doubling my money for that uncertainty. They also made the point to mention the Citizens Petition as not resolved in the PR. Again, more uncertainty. Feels weird being out of MNKD after 10 years of being in, but there was too much risk in the short term and too many other opportunities to make money while we wait for UTHR and MNKD to communicate what is really going on, the real steps to fix it and how long. So far they have said next summer, which tells me it’s not an easy fix. |
|
|
|
Post by Clement on Oct 23, 2021 7:50:00 GMT -5
Quote: "United Therapeutics will be moving the treprostinil testing to its own facility, Castagna said, and will file again with the FDA. The company originally filed its new drug application in April." My thoughts: What has to be done at UTHR to do this testing in-house? If UTHR could already do it, they wouldn't have been paying an outside lab. So they possibly have to allocate space, buy or reassign instrumentation, install the instrumentation, train technicians, set up the SOP for the procedure, reapply and get an FDA inspection on the new test. Now I see why this will take seven months or so. Hopefully, they can get the space allocated and instrumentation set up before Christmas. |
|
|
|
Post by porkini on Oct 23, 2021 8:50:10 GMT -5
Quote: "United Therapeutics will be moving the treprostinil testing to its own facility, Castagna said, and will file again with the FDA. The company originally filed its new drug application in April." My thoughts: What has to be done at UTHR to do this testing in-house? If UTHR could already do it, they wouldn't have been paying an outside lab. So they possibly have to allocate space, buy or reassign instrumentation, install the instrumentation, train technicians, write the SOP for the procedure, reapply and get an FDA inspection on the new test. Now I see why this will take seven months or so. Hopefully, they can get the space allocated and instrumentation set up before Christmas. Conflict of interest, accountability is all in-house, checks and balances? I don't know anything, just what I would think. |
|
|
|
Post by prcgorman2 on Oct 23, 2021 9:06:13 GMT -5
It’s a nit, but “own facility” and “in-house” are not exactly the same thing (although they likely are). I do not assume that UTHR was blind-sided (although maybe they were), nor do I assume that they are not willing to throw money at as rapid a solution as can be put in place. I’ve not studied how often 2nd CRLs happen but from this board and other places I’ve read I get the feeling they are not common meaning there’s a better than 50/50 chance (back to odds again) that UTHR will get things sorted out satisfactorily and hit close to the dates they’re saying. I also get the feeling that Dr. Rothblatt likes to set and achieve expectations and is too shrewd to be overly aggressive on setting expectations. All that to say I think the moves are more likely to be based on planning than scrambling.
|
|
|
|
Post by markado on Oct 23, 2021 9:07:10 GMT -5
Conflict of interest, accountability is all in-house, checks and balances? I don't know anything, just what I would think. There is no conflict of interest. It is quite normal and acceptable for pharma companies to test there own Rx API in house. That's the whole purpose and premise of GMP. That UTHR had an outside lab performing this test, to begin with, may have been a cost, convenience, capacity decision at the time. But to in-source this activity after the FDA's finding at the third party facility is likely to better ensure control, compliance and outcome. The method of testing is likely already established and approved. It's just a question of re-housimg and replicating the execution of the test method, and that may take a couple to few months to sort out. My guess, though, is that process was already in motion prior to the formal receipt if the CRL. But, that's just a guess, based on the same overall review that allowed MC to know there was a small issue in Danbury, months ago. Given what is at stake, I don't see Martine as drumming fingers on a desktop trying to decide what to do. |
|
|
|
Post by prcgorman2 on Oct 23, 2021 9:13:03 GMT -5
Conflict of interest, accountability is all in-house, checks and balances? I don't know anything, just what I would think. There is no conflict of interest. It is quite normal and acceptable for pharma companies to test there own Rx API in house. That's the whole purpose and premise of GMP. That UTHR had an outside lab performing this test, to begin with, may have been a cost, convenience, capacity decision at the time. But to in-source this activity after the FDA's finding at the third party facility is likely to better ensure control, compliance and outcome. The method of testing is likely already established and approved. It's just a question of re-housimg and replicating the execution of the test method, and that may take a couple to few months to sort out. My guess, though, is that process was already in motion prior to the formal receipt if the CRL. But, that's just a guess, based on the same overall review that allowed MC to know there was a small issue in Danbury, months ago. Given what is at stake, I don't see Martine as drumming fingers on a desktop trying to decide what to do.I couldn’t give more than one thumbs up but your post is helpfully informative and your last sentence especially resonates with me. |
|
|
|
Post by peppy on Oct 23, 2021 9:54:02 GMT -5
There is no conflict of interest. It is quite normal and acceptable for pharma companies to test there own Rx API in house. That's the whole purpose and premise of GMP. That UTHR had an outside lab performing this test, to begin with, may have been a cost, convenience, capacity decision at the time. But to in-source this activity after the FDA's finding at the third party facility is likely to better ensure control, compliance and outcome. The method of testing is likely already established and approved. It's just a question of re-housimg and replicating the execution of the test method, and that may take a couple to few months to sort out. My guess, though, is that process was already in motion prior to the formal receipt if the CRL. But, that's just a guess, based on the same overall review that allowed MC to know there was a small issue in Danbury, months ago. Given what is at stake, I don't see Martine as drumming fingers on a desktop trying to decide what to do.I couldn’t give more than one thumbs up but your post is helpfully informative and your last sentence especially resonates with me. I am guessing, I do not know enough..... A sealed off floor or level whatever lab with bots? Is it testing that is being brought in house or manufacturing? 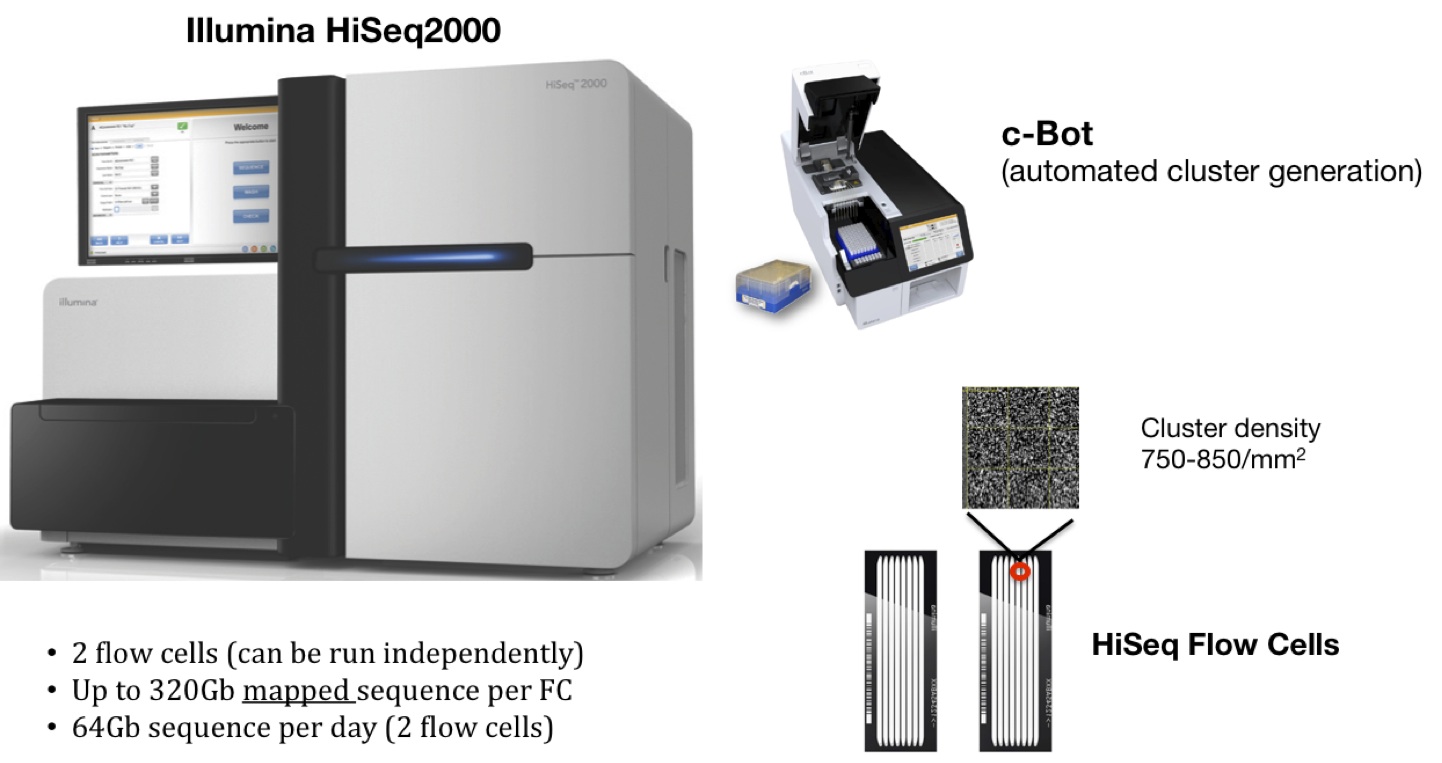  |
|
|
|
Post by Clement on Oct 23, 2021 12:21:19 GMT -5
There is more instrumentation in heaven and earth than drempt of in your philosophies, pepita.
(As you can see I like to quote Shakespeare -- with a twist.)
|
|
|
|
Post by Clement on Oct 23, 2021 12:23:31 GMT -5
Conflict of interest, accountability is all in-house, checks and balances? I don't know anything, just what I would think. There is no conflict of interest. It is quite normal and acceptable for pharma companies to test there own Rx API in house. That's the whole purpose and premise of GMP. That UTHR had an outside lab performing this test, to begin with, may have been a cost, convenience, capacity decision at the time. But to in-source this activity after the FDA's finding at the third party facility is likely to better ensure control, compliance and outcome. The method of testing is likely already established and approved. It's just a question of re-housimg and replicating the execution of the test method, and that may take a couple to few months to sort out. My guess, though, is that process was already in motion prior to the formal receipt if the CRL. But, that's just a guess, based on the same overall review that allowed MC to know there was a small issue in Danbury, months ago. Given what is at stake, I don't see Martine as drumming fingers on a desktop trying to decide what to do. I like the emboldened comment above. Martine did say summer "or earlier". |
|
|
|
Post by ktim on Oct 24, 2021 14:12:46 GMT -5
It’s a nit, but “own facility” and “in-house” are not exactly the same thing (although they likely are). I do not assume that UTHR was blind-sided (although maybe they were), nor do I assume that they are not willing to throw money at as rapid a solution as can be put in place. I’ve not studied how often 2nd CRLs happen but from this board and other places I’ve read I get the feeling they are not common meaning there’s a better than 50/50 chance (back to odds again) that UTHR will get things sorted out satisfactorily and hit close to the dates they’re saying. I also get the feeling that Dr. Rothblatt likes to set and achieve expectations and is too shrewd to be overly aggressive on setting expectations. All that to say I think the moves are more likely to be based on planning than scrambling. Second CRL in general or with respect to MNKD? lol With respect to MNKD 2nd CRLs statistically speaking are 100%. |
|