|
|
Post by peppy on May 21, 2022 5:57:53 GMT -5
shawnonafrezza I found it. You did say you take regular insulin. "Just an update since I said I was getting labs and this is what would be possible with a different soc... Tresiba + R + Afrezza imgur.com/a/m4zlFT1 This was not expected at all. Dexcom estimated much higher." 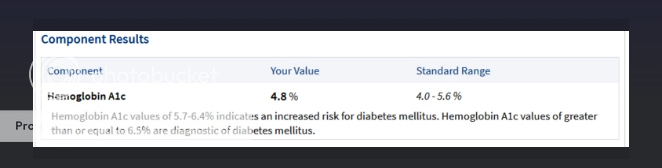 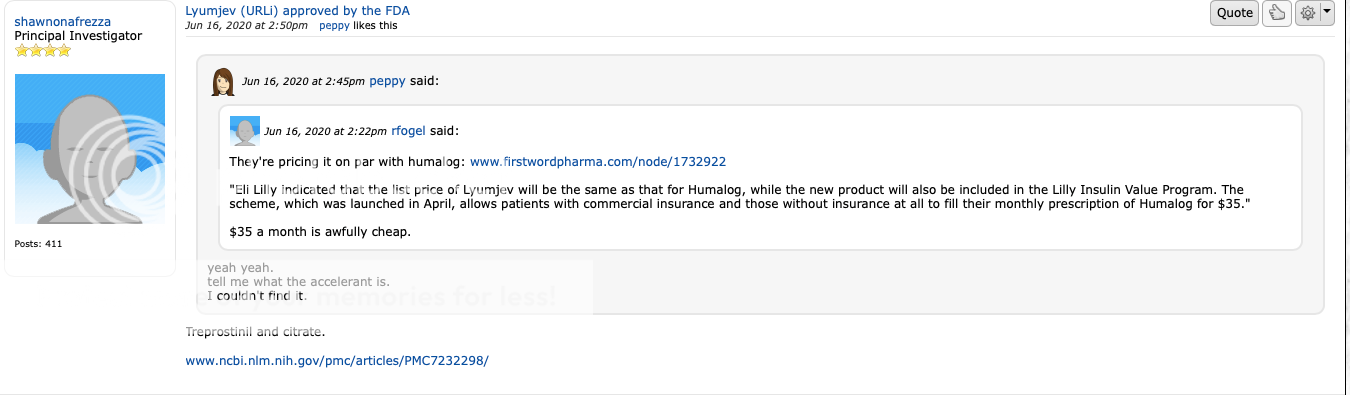 |
|
|
|
Post by sayhey24 on May 21, 2022 8:14:44 GMT -5
...Now that Dave Kendall is back in the picture and Mounjaro is approved maybe we can get him back too and he can restart his SoC efforts. The $10M would be much better spent hiring those two guys rather than the 16 sales reps. So with all these negatives and critiques and predictions of failure, last weeks revenues were at an all week high and the tend is clearly in a steep up ramp and income streams are multiplying. Go figure., [/quote] Its not surprising afrezza sales are up and they will continue to grow in the T1 market as more and more T1s share their experiences and success. Top T1 "thought leaders" like Steve Edelman and Gary Scheiner and even the top guy at the ADA use afrezza and have spoken of its benefits. Now that Ginger Vieira has rediscovered afrezza she has numerous positive articles on beyondtype1.org You read a story like this an you wonder why more T1s are not using beyondtype1.org/inhaled-insulin-type-1-diabetes/But lets remember the T1 trial was terrible and most never even thought afrezza would be approved for T1s. If you dose according to the label you probably wont see the true benefits of afrezza and may even say afrezza does not work for you. However as word of mouth support builds in the T1 world so does afrezza sales and it will continue to grow but just not at the blockbuster rate which Technosphere Insulin has. Its potential will not be realized. With that said lets step back and think about things. "afrezza" has always had two distinct markets; the T1s; and the T2s. They are very different and even required different trials. Maybe two separate new drug applications should have been filed and two separate drug should have been created based on Technosphere Insulin (TI); one for T1s; and one for T2s. Back in the day everyone was hoping the ADA would recognized a new "Class" for afrezza as "ultra rapid acting" insulin. We already have RAA - rapid acting analogs and afrezza can not be in that class as its not an analog. But for whatever reason we got the bland "inhaled insulin" and we know all "inhaled insulins" are not the same. Exubera can not do what afrezza does. Over time and with things like the PEDS trial afrezza use in the T1 market will grow. However the big market for afrezza is in the T2 market just not as a mealtime insulin. It needs to target SoC step 2. When Sanofi licensed afrezza they did so with the T2 market as their target. The Sanofi guy heading it up saw it as a direct competitor to the GLP1s and SGLT2s. He was not interested in afrezza as "an insulin" but rather as a GLP1 replacement. At this point most attempts MNKD have tried into the T2 market have been an epic fail and will continue to be an epic fail until MNKD executes on the roadmap Sanofi put together but never executed. If you really want to see Technosphere Insulin become a blockbuster MNKD needs a new product with a new name in a new ADA class, with completely different dosing directs and targeting the GLP1s. |
|
|
|
Post by sportsrancho on May 21, 2022 8:39:24 GMT -5
From what I heard from the reps, Kendall was “spinning his wheels” and not getting anywhere.
|
|
|
|
Post by cjm18 on May 21, 2022 9:25:49 GMT -5
From what I heard from the reps, Kendall was “spinning his wheels” and not getting anywhere. Doubt anything has changed since he left. If he couldn’t get the ball rolling without the peds trial then no one could. |
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on May 21, 2022 9:43:16 GMT -5
From what I heard from the reps, Kendall was “spinning his wheels” and not getting anywhere. It's to be expected. There is NO WAY the ADA or anyone is going to include Afrezza in the SOC. The BIG 3 will do everything in their power to squash it? Kendall probably thought he had enough clout to get it done. There is too much influence and power controlled by the BIG 3. The only way it will happen is after the PEDS Approval and MNKD's NEW PARTNER will initiate it. And that NEW PARTNER will have to be one of the BIG 3. |
|
|
|
Post by letitride on May 21, 2022 13:10:25 GMT -5
If the PEDS trial proves to be superior without sticking needles in these kids three and four times a day and allows kids to be kids. I believe the big3 will buyout or get out the way. Either way its a big win for all.
|
|
|
|
Post by wesnigon on May 21, 2022 20:16:23 GMT -5
The V-GO also has a nice ring for a formula one car. I can't believe no one connected the dots on that. Anyways, my birthday is Wednesday, hoping for a nice return on my investment one of these days.
|
|
|
|
Post by prcgorman2 on May 22, 2022 10:37:53 GMT -5
I had thought more like Viggo as in Viggo Mortensen. But maybe that is what the name means? We don’t sit idle, V-Go!
|
|
|
|
Post by agedhippie on May 22, 2022 10:50:38 GMT -5
 agedhippie agedhippie , any chance, a closed loop system? am I off the rocker, totally misunderstanding? ... Reading the thread, yes that's a closed loop system - he is eating without telling the APS and letting it realize that and handle it. The APS gets feedback from the CGM and corrects to bring him to the target range so it doesn't care if the spike comes from food or stress, it will deal with it without bothering him. The choice of pizza makes the task easier as it is not spiky since it is slow carbs. There is a comment that it struggles to stop spikes over 180 with rapid carbs as you are bolusing after the event, and it uses small bolus doses for safety. It looks like the AAPS ( androidaps.readthedocs.io/en/latest/Getting-Started/WhatisAndroidAPS.html) from comments, and that is designed as a closed loop system from the DIY APS community. Two points of interest; a faster pumpable insulin would make this better (they are using Lyumjev rather than a traditional RAA), and they are using U-200 to improve absorption. The latter is interesting as it is what I am doing now but with Humalog (I am still wary of the incipients in these new insulins) as the smaller dose gives a greater ratio of surface area to volume. |
|
|
|
Post by peppy on May 22, 2022 10:58:12 GMT -5
agedhippie Can you come up with some possible reasons Mike may have bought the dumb pump from Kendall for 10 million besides a customer/revenue theory presented? MNKD Happens to have regular insulin and Treprostinil
|
|
|
|
Post by agedhippie on May 22, 2022 11:18:07 GMT -5
agedhippie Can you come up with some possible reasons Mike may have bought the dumb pump from Kendall for 10 million besides a customer/revenue theory presented? MNKD Happens to have regular insulin and Treprostinil Mannkind's focus is on inhaled drugs so an injectable device doesn't make a lot of sense unless there is a desire to branch beyond just inhaled drugs into inhaled,/ slow injection bundles. For diabetes I can't see any reason that makes sense since pens are going to win every time for basal - I can inject once a day (soon to be once a week) or I can walk around with this thing glued to me and have to swap it every day. |
|
|
|
Post by agedhippie on May 22, 2022 11:36:19 GMT -5
I was curious as to how the V-Go purchase was being described so I went back to the Mannkind PR since that's their pitch to the market.
"The acquisition of V-Go allows MannKind to expand its portfolio and strengthen its commitment to providing innovative mealtime diabetes solutions."
"Designed to be patient-friendly, V-Go is worn like a patch and eliminates the need for taking multiple daily shots."
"MannKind is passionate about being a leader in mealtime control to address this unmet need within the diabetes community," (Mike)
It feels like they are going to run this the same way it has been up until now, as a combined basal and mealtiime device, and as an alternative to Afrezza. If someone is using this and is happy they are unlikely to make changes. This is a device that boluses in 2u chunks of RAA so it's primary focus has to be convenience - nothing loose to carry around and that market is not going to be particularly open to the idea of Afrezza which involves more work and carrying stuff around.
I think Mike sees it as a low risk purchase. The cost can be defrayed by milking the existing users and if it all goes wrong it's only $10M and they have an approved mechanical syringe which may be useful down the road. I am not sure it will be cashflow positive after all costs, but it's probably close enough to be a wash.
|
|
|
|
Post by sayhey24 on May 22, 2022 15:11:00 GMT -5
Aged - Mike was on a "talk" the same day it was announced. Its probably still available as a podcast. If you listen to it he basically said what you said. He explains the purchase.
He did not address the issue that if you are a sales guy and really understand and believe in afrezza these are competing products. Maybe he does not see it that way but I would like to take him to dinner and hear his side. To me its a boneheaded purchase and there are much better things to focus on which may really move the ball.
|
|
|
|
Post by sayhey24 on May 22, 2022 15:18:34 GMT -5
 agedhippie agedhippie , any chance, a closed loop system? am I off the rocker, totally misunderstanding? ... Reading the thread, yes that's a closed loop system - he is eating without telling the APS and letting it realize that and handle it. The APS gets feedback from the CGM and corrects to bring him to the target range so it doesn't care if the spike comes from food or stress, it will deal with it without bothering him. The choice of pizza makes the task easier as it is not spiky since it is slow carbs. There is a comment that it struggles to stop spikes over 180 with rapid carbs as you are bolusing after the event, and it uses small bolus doses for safety. It looks like the AAPS ( androidaps.readthedocs.io/en/latest/Getting-Started/WhatisAndroidAPS.html) from comments, and that is designed as a closed loop system from the DIY APS community. Two points of interest; a faster pumpable insulin would make this better (they are using Lyumjev rather than a traditional RAA), and they are using U-200 to improve absorption. The latter is interesting as it is what I am doing now but with Humalog (I am still wary of the incipients in these new insulins) as the smaller dose gives a greater ratio of surface area to volume. Aged - this is where Al Mann was headed. He was looking for a faster insulin to put in his pumps. I think we are at about the limits of pumps and algorithms until there is a faster insulin and thats really hard because you have variables like insulin absorption. Afrezza on the other handle simplifies the mealtime spike and is very consistent. The pump can then do its thing. The AP and afrezza are not competitive devices. If the AP is used correctly and programmed for afrezza they can be complimentary. That will be the next big thing - programming the AP for use with afrezza. |
|
|
|
Post by sayhey24 on May 22, 2022 16:24:39 GMT -5
From what I heard from the reps, Kendall was “spinning his wheels” and not getting anywhere. It's to be expected. There is NO WAY the ADA or anyone is going to include Afrezza in the SOC. The BIG 3 will do everything in their power to squash it? Kendall probably thought he had enough clout to get it done. There is too much influence and power controlled by the BIG 3. The only way it will happen is after the PEDS Approval and MNKD's NEW PARTNER will initiate it. And that NEW PARTNER will have to be one of the BIG 3. Lets not conflate the ADA T1 SoC and the T2 SoC. The are different. Additionally the T1 market and the T2 markets are different and should not be conflated. Casper - you may be correct and it may require a BP to get afrezza in its proper place in the ADA T2 SoC. When I say proper place I mean as a new ADA "Class" for the replacement of GLP1s. Sports - Maybe the Sales Reps are correct and maybe not. While I am pretty critical of Dave Kendall for "leaving us flat" he did get some mention of afrezza in the T1 SoC. I think we need to give Dave credit for these words in section 9 - "Inhaled human insulin has a rapid peak and shortened duration of action compared with RAA and may cause less hypoglycemia and weight gain (8)" These Sales Reps should be able to do something with that. While its clearly not perfect and needs some rework it was a start. It gives them an opening in the T1 world. Calling Technosphere Insulin "Inhaled Insulin" is a none starter. Exubera and afrezza are completely different. What happens if that other thing which looks like a Super 8 movie camera gets approved - does that automatically inherit those words without a head to head trial with afrezza? Afrezza is also in the rapid insulin class. This is clearly not were it belongs. It needs its own class. This "Class" would also need to be changed from "inhaled insulin" to something else. For the T1 SoC I would be OK with Ultra Acting or Near normal but the class can't be Rapid Acting where it is now. Maybe Dave thought he could fix this later. Why Dave left I have no idea. We know Duane DeSisto was told by Insulet not to come. In Dave's case he was making progress and then POOF - gone. The easiest job he ever had he leaves. He makes some progress with the T1 SoC and I would think he was now targeting the T2 SoC. At the same time Lillly is working to get Mounjaro trails complete and the last thing they would want is Dave coming out with a new class beating the GLP1s. Did Dave also have a non compete with Lilly? If so that would explain the sudden exit and no word from Mike that I can remember. At the time I thought maybe it was a personal issue. It may also explain the sudden appearance again now that Mounjaro is approved. The more I think about that $10M and how it should have been spent on a head to head trial with Mounjaro the more my head wants to explode. I sure hope Mike leverages what he has in the T1 SoC and does some great "Seeing is Believing" demos at ADA next week. He better do some demos on some T2s and non-diabetics taking afrezza and not getting hypos. The non-hypo demo is key for the Mounjaro trial. I would also suggest he leaves the V-Go at home and tries to figure out how to get out of the deal. |
|