Deleted
Deleted Member
Posts: 0
|
Post by Deleted on May 26, 2022 0:18:23 GMT -5
So Clofazimine is a drug indicated for Leprosy. Apparently it can be repurposed for Non-Tuberculosis Mycobacterium disease (NTM) which is a bacteria that enters the lung and without treatment can cause permanent damage to the lungs.
Since Clofazimine is not labeled for NTM will MNKD have to perform extra trials to show safety and efficacy?
Do we know who (if anyone) owns Clofaznimine? I went back and looked at MNKDs progression of TreT and they entered into a Phase 1 Study in March 2018 and it was completed 90 days later. 90 days after that MNKD signed an agreement with UTHR. So start to finish was 6 months.
Now do you think Clofazimine will take the same path or will additional trials be necessary?
I guess MNKD will have discussions with the FDA on the proper path......505(b)(2)??
Any thoughts???
|
|
|
|
Post by buyitonsale on May 26, 2022 2:03:13 GMT -5
|
|
|
|
Post by Clement on May 26, 2022 6:49:17 GMT -5
phase 1 -- SAD part is finished and MAD top lines available in Q3
The following is from Q1 earnings call:
On MNKD-101, which is our clofazimine product, we have completed Part A yesterday. Thank God. So we have done cohorts A, 1A2 and A3 leave out those patients up to 90 milligrams, which is the highest dose we thought we should go. And we've so far have seen great tolerability with no safety signals.
We'll now wait for the full data set to be analyzed along with PK and PD and assess the proper doses here for Part B. Well now, on the low end, we'll go to 30 milligrams, which we think is more than enough to overcome any MICs. And we are debating whether to go to 90 or 60 based on the data coming out of Part A. We think we can go to 90 and it would probably make sense to go to 90, just to have the max tolerated dose.
Top lines are expected here in Q3 from the MAD part, obviously in Part A, we know the safety and tolerability of clofazimine looks very strong in the nebulized formulation.
|
|
|
|
Post by peppy on May 26, 2022 7:25:47 GMT -5
phase 1 -- SAD part is finished and MAD top lines available in Q3 The following is from Q1 earnings call: On MNKD-101, which is our clofazimine product, we have completed Part A yesterday. Thank God. So we have done cohorts A, 1A2 and A3 leave out those patients up to 90 milligrams, which is the highest dose we thought we should go. And we've so far have seen great tolerability with no safety signals. We'll now wait for the full data set to be analyzed along with PK and PD and assess the proper doses here for Part B. Well now, on the low end, we'll go to 30 milligrams, which we think is more than enough to overcome any MICs. And we are debating whether to go to 90 or 60 based on the data coming out of Part A. We think we can go to 90 and it would probably make sense to go to 90, just to have the max tolerated dose. Top lines are expected here in Q3 from the MAD part, obviously in Part A, we know the safety and tolerability of clofazimine looks very strong in the nebulized formulation. This years graphic followed by last years graphic. 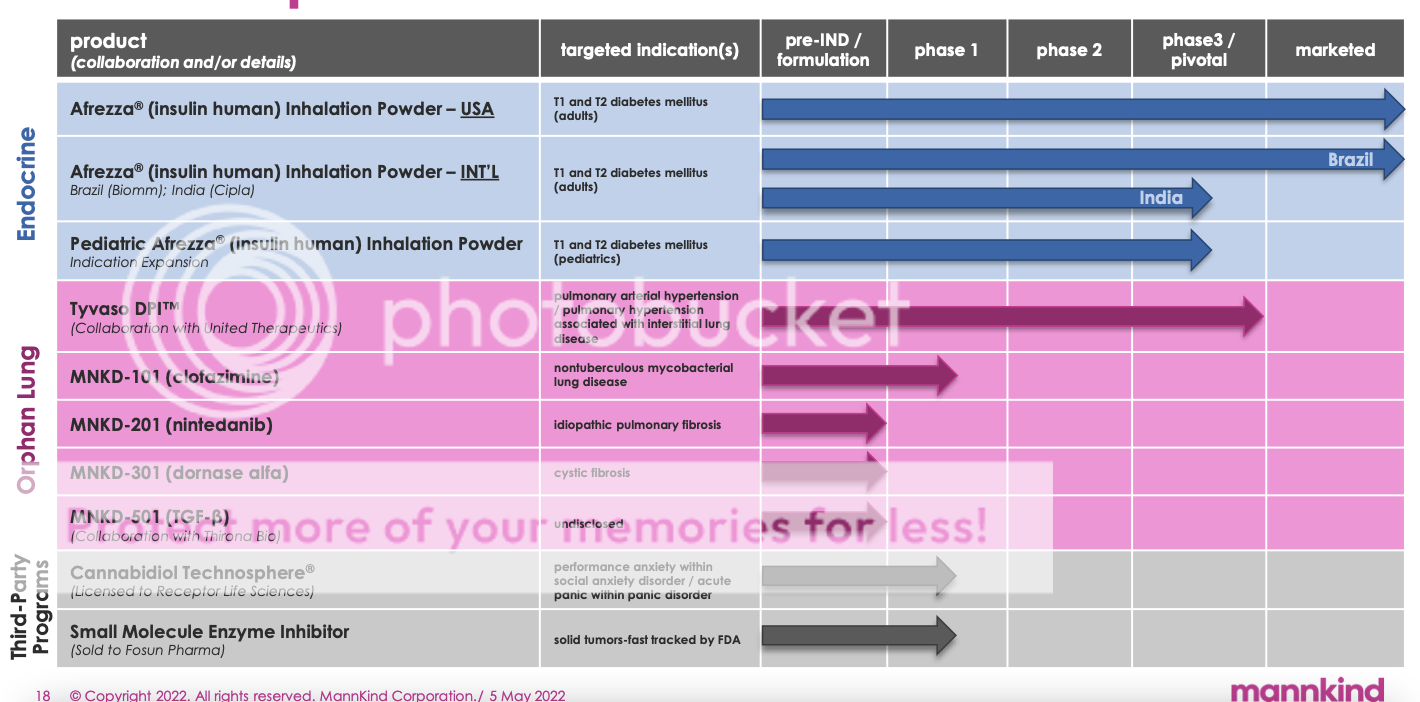   |
|
|
|
Post by hellodolly on May 26, 2022 7:56:30 GMT -5
peppy My guess we hear something about further development on MNKD501, before the end of June. Under the terms of the collaboration, which began in June 2021, the companies are evaluating the therapeutic potential of Thirona’s locally acting TGF-β inhibitor, FBM5712, for the treatment of pulmonary fibrosis. MannKind is developing MNKD-501, a dry powder inhaled formulation of FBM5712, which is advancing to a nonclinical pharmacodynamics (PD) study with key results anticipated in 2Q 2022. If initial studies are promising, MannKind can exercise certain rights to seek a full license to the compound for clinical development and commercialization for the treatment of fibrotic pulmonary diseases.
|
|
|
|
Post by LongMNKD on May 26, 2022 9:34:38 GMT -5
Speaking on MNKD501 - It is indicated for pulmonary fibrosis. But Treprostinil is also trying to expand it's indication to pulmonary fibrosis? ![]()  Would they be competing for the same market? |
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on May 26, 2022 10:53:48 GMT -5
Speaking on MNKD501 - It is indicated for pulmonary fibrosis. But Treprostinil is also trying to expand it's indication to pulmonary fibrosis? ![]()  Would they be competing for the same market? I think you mean MNKD-201 |
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on May 26, 2022 10:59:08 GMT -5
Speaking on MNKD501 - It is indicated for pulmonary fibrosis. But Treprostinil is also trying to expand it's indication to pulmonary fibrosis? ![]()  Would they be competing for the same market? I think you mean MNKD-201 It's possible UTHR and Boehringer Ingelheim Pharmaceutical will be competing in the IPF indication. That's the beauty of MNKD.....They take no sides. And I'm sure the IPF market is big enough for more than one player. Mike said the other day the COPD & IPF market is > 4x the PAH market. It's huge. |
|
Deleted
Deleted Member
Posts: 0
|
Post by Deleted on May 26, 2022 11:07:24 GMT -5
I think you mean MNKD-201 It's possible UTHR and Boehringer Ingelheim Pharmaceutical will be competing in the IPF indication. That's the beauty of MNKD.....They take no sides. And I'm sure the IPF market is big enough for more than one player. Mike said the other day the COPD & IPF market is > 4x the PAH market. It's huge. FDA approves Ofev® as first treatment for chronic fibrosing ILDs with a progressive phenotype This FDA approval is the third for Ofev® (nintedanib), first approved for idiopathic pulmonary fibrosis (IPF) in 2014 Ofev slowed lung function decline by 57 percent relative to placebo across overall study population as assessed by the annual rate of decline in forced vital capacity in the Phase III INBUILD® trial Chronic fibrosing ILDs in which lung fibrosis continues to worsen include a large group of diseases that may lead to a serious pulmonary condition with irreversible scarring of lung tissue |
|
|
|
Post by peppy on May 26, 2022 11:12:50 GMT -5
|
|
|
|
Post by markado on May 26, 2022 11:52:40 GMT -5
Plus, and I'm just speaking off the cuff here, I believe MNKD Tyvaso DPI was able to provide equal to better benefits with less API. All physicians tend to agree that the less medicine needed to achieve the same or better benefit, the better. Maybe the same will prove true of most, if not many of the APIs combined with Technosphere. If so, efficacy will be a key comparitive measure and positive differentiator going forward.
|
|
|
|
Post by mango on May 26, 2022 15:08:19 GMT -5
I wonder whose nebulizer we are using and who is manufacturing the solution…
|
|