|
|
Post by prcgorman2 on Sept 25, 2022 10:22:04 GMT -5
... To me, the best argument left for giving away starter packs of CGM sensors and subsidizing their cost, then, is from insurance companies who will make up the difference in lowered cost of outcomes, but that's a whole other discussion. At this point you run into the short term view of the US insurer. On average you change jobs every four years and with that change often change insurers. Since we are talking about T2 here then there is little risk of ER levels of hypoglycemia so the complications are all at the long term end - far beyond that four year window. Fifteen years down the line when complications are starting they are probably on Medicare now and the government's problem. The problem is that the insurers will only adopt measures that are either required by the SoC, or will save them money in the immediate term. Insurers are for-profit companies and have a legal obligation to put the interests of their shareholders before those of their customer/patients. As I said, insurance is another discussion. Very interesting insight agedhippie. You’ve shared before your views about the short window, relatively, that insurance underwriters and actuarials are focused on. But this time you added the additional tidbit about T2s not being as big a concern for ER visits, and that they’re often later in adult life and more of a problem for medicare (and I’ll throw in medicaid) than for insurers. You are persuasive. And your comments are encouraging. The government is run by <———>, er, I mean politicians, who are persuaded to care about anything they think their constituents or lobbyists care about. They care about constituents insofar as their votes should be needed to get elected, and they care about lobbyists who can organize to provide campaign money and hire policy attorneys and subject matter experts to inform and influence the views of politicians. Thus, the good thing the Reddit and motivated investors can do is organize an effort to get politicians to get medicare to underwrite CGMs like candy to improve outcomes and reduce long term cost of care for diabetes comorbidities. |
|
|
|
Post by cretin11 on Sept 25, 2022 17:59:07 GMT -5
aged is spot on with those thoughts on the insurance industry, its loyalty to shareholders over patients/insureds and its short term perspective on payouts. And when it comes to affecting the legislative process, it’s good to keep in mind the insurance industry is one of the top two or three in terms of $$ power (big pharma being maybe the only larger lobby). Laws ain’t changing without those lobbies having their fingerprints all over it, sadly there’s no amount of reddit activity that can effect meaningful change to that dynamic.
|
|
|
|
Post by prcgorman2 on Sept 26, 2022 6:48:10 GMT -5
No disagreement although I didn’t suggest a change in any existing law or say there needs to be a new one. Government agencies are influenced by their administration which is influenced by politicians. Letters, inquisitions, discussions with secretaries, are all influential when it comes to making policy based on political motivations. If enough of the right politicians get it in their head that CGMs are popular and relatively unavailable due to lack of the right policy, the policy can get modified without a new law. I consider DXCM and other CGM manufacturers part of pharma and able to lobby. Now, if big pharma perceives that as a threat, it may not do them any good considering how often they appear on the wrong side of what is popular with voters.
|
|
|
|
Post by mango on Sept 27, 2022 10:41:55 GMT -5
Some very interesting and insightful thoughts regarding the T2D market.
I think a large scale trial with Afrezza should be done for this patient population. Preferably using a CGM and go head-to-head (not a superiority trial) with Step 1 therapy for T2D. Primary outcomes should be TIR, glucose excursions and post prandial hyperglycemia. Afrezza participates in the trial receive 2 weeks of coaching with the use of Afrezza and CGM as well as the Bluhale device utilization throughout the entire study for monitoring and intermediate adjustments if necessary in patient’s inhalation technique and/or timing/dose.
400+ participants, 26 weeks.
|
|
|
|
Post by agedhippie on Sept 28, 2022 17:50:24 GMT -5
Some very interesting and insightful thoughts regarding the T2D market. I think a large scale trial with Afrezza should be done for this patient population. Preferably using a CGM and go head-to-head (not a superiority trial) with Step 1 therapy for T2D. Primary outcomes should be TIR, glucose excursions and post prandial hyperglycemia. Afrezza participates in the trial receive 2 weeks of coaching with the use of Afrezza and CGM as well as the Bluhale device utilization throughout the entire study for monitoring and intermediate adjustments if necessary in patient’s inhalation technique and/or timing/dose. 400+ participants, 26 weeks. The problem with a head to head for T2 is that you are looking at a multi-year, very large scale trial. As an example UKPDS was 5,000+ people for 20 years, VADT was 1,800 people for 15 years, both these trials are in run-on phases now with UKPDS being 45 years old now. Usually only government organizations can afford to fund these (UKPDS was started in 1977 is funded by the NHS, VADT started in 2002 and is funded by the Veterans Administration) These are the trials that form opinion as they have the scale and longitudinal time to show long term outcomes for treatments. |
|
|
|
Post by beardawg on Oct 3, 2022 16:28:06 GMT -5
Some very interesting and insightful thoughts regarding the T2D market. I think a large scale trial with Afrezza should be done for this patient population. Preferably using a CGM and go head-to-head (not a superiority trial) with Step 1 therapy for T2D. Primary outcomes should be TIR, glucose excursions and post prandial hyperglycemia. Afrezza participates in the trial receive 2 weeks of coaching with the use of Afrezza and CGM as well as the Bluhale device utilization throughout the entire study for monitoring and intermediate adjustments if necessary in patient’s inhalation technique and/or timing/dose. 400+ participants, 26 weeks. The problem with a head to head for T2 is that you are looking at a multi-year, very large scale trial. As an example UKPDS was 5,000+ people for 20 years, VADT was 1,800 people for 15 years, both these trials are in run-on phases now with UKPDS being 45 years old now. Usually only government organizations can afford to fund these (UKPDS was started in 1977 is funded by the NHS, VADT started in 2002 and is funded by the Veterans Administration) These are the trials that form opinion as they have the scale and longitudinal time to show long term outcomes for treatments. The UKPDS trial was determining whether therapies are effective. The trial Mango is proposing is not asking whether something is effective. That takes time. His trial isn't introducing something new; it's measuring the same things that have always been measured and known to be effective, and now just actually specifically looking at a part of it that. Mango's trial would be like a piggy back off UKPDS as a small follow-up trial. UKPDS says reducing blood glucose levels reduces how much damage is done. Now that we know that, we can do Mango's trial to determine if current initial SOC actually holds BG levels in check all the time like insulin potentially does (and Afrezza does). It doesn't take long to see that, and doesn't take nearly as many participants. You're measuring short term outcomes, so the datapoints that were gathered daily with UKPDS would be gathered minute by minute with mango's trial; you're measuring over days, not years. We already know that excursions outside of the normal range can be detrimental (acute - hypos, chronic - further damage) |
|
|
|
Post by agedhippie on Oct 4, 2022 9:32:12 GMT -5
The UKPDS trial was determining whether therapies are effective. The trial Mango is proposing is not asking whether something is effective. That takes time. His trial isn't introducing something new; it's measuring the same things that have always been measured and known to be effective, and now just actually specifically looking at a part of it that. Mango's trial would be like a piggy back off UKPDS as a small follow-up trial. UKPDS says reducing blood glucose levels reduces how much damage is done. Now that we know that, we can do Mango's trial to determine if current initial SOC actually holds BG levels in check all the time like insulin potentially does (and Afrezza does). It doesn't take long to see that, and doesn't take nearly as many participants. You're measuring short term outcomes, so the datapoints that were gathered daily with UKPDS would be gathered minute by minute with mango's trial; you're measuring over days, not years. We already know that excursions outside of the normal range can be detrimental (acute - hypos, chronic - further damage) The question that is being raised is if bolus insulin should be used as a first step for Type 2. That's not something that has not been addressed so far in UKDPS so there is not data set. What they would be looking to quantify whether any extra benefits of tighter control from insulin is meaningful over the long term and can be sustained vs. starting on metformin and transitioning later. The data set exists for the latter, but not for the former. What you are suggesting is more of a TIR trial, what UKPDS (and the SoC) is long term outcomes. The question a UKPDS type trial would ask is does TIR matter in the long term and how much. Once that is answered then you could piggyback with the Afrezza TIR data. Right now there is long longitudinal TIR data and that is a problem that they are working to fix. |
|
|
|
Post by harryx1 on Oct 4, 2022 10:05:47 GMT -5
|
|
|
|
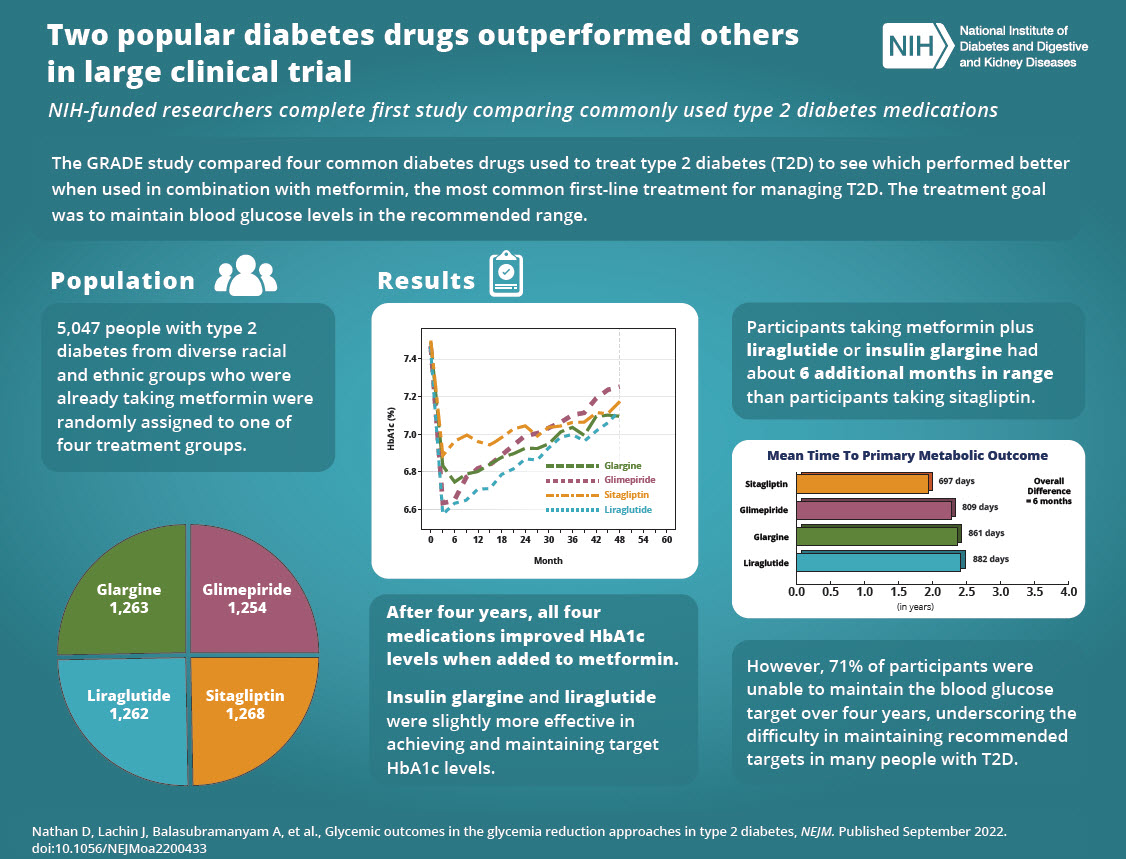
Post by akemp3000 on Oct 4, 2022 11:07:27 GMT -5
Thanks Harry! The text in the lower right corner speaks volumes regarding the failure of the current Standards of Care.
|
|
|
|
Post by peppy on Oct 4, 2022 11:42:45 GMT -5
From Harry's post two posts above. Insulin glargine and liraglutidewere slightly more effective in achieving and maintaining target HbA1c levels. ========================================================================= www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdfVICTOZA® (liraglutide) injection, for subcutaneous use Initial U.S. Approval: 2010 WARNING: RISK OF THYROID C-CELL TUMORS See full prescribing information for complete boxed warning. • Liraglutide causes thyroid C-cell tumors at clinically relevant exposures in both genders of rats and mice. It is unknown whether VICTOZA causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of liraglutide-induced rodent thyroid C-cell tumors has not been determined (5.1, 13.1). • VICTOZA is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk of MTC and the symptoms of thyroid tumors (4, 5.1). |
|
|
|
Post by beardawg on Oct 4, 2022 13:32:17 GMT -5
The UKPDS trial was determining whether therapies are effective. The trial Mango is proposing is not asking whether something is effective. That takes time. His trial isn't introducing something new; it's measuring the same things that have always been measured and known to be effective, and now just actually specifically looking at a part of it that. Mango's trial would be like a piggy back off UKPDS as a small follow-up trial. UKPDS says reducing blood glucose levels reduces how much damage is done. Now that we know that, we can do Mango's trial to determine if current initial SOC actually holds BG levels in check all the time like insulin potentially does (and Afrezza does). It doesn't take long to see that, and doesn't take nearly as many participants. You're measuring short term outcomes, so the datapoints that were gathered daily with UKPDS would be gathered minute by minute with mango's trial; you're measuring over days, not years. We already know that excursions outside of the normal range can be detrimental (acute - hypos, chronic - further damage) That's not something that has not been addressed so far in UKDPS so there is not data set. What they would be looking to quantify whether any extra benefits of tighter control from insulin is meaningful over the long term and can be sustained vs. starting on metformin and transitioning later. The data set exists for the latter, but not for the former. What you are suggesting is more of a TIR trial, what UKPDS (and the SoC) is long term outcomes. The question a UKPDS type trial would ask is does TIR matter in the long term and how much. Once that is answered then you could piggyback with the Afrezza TIR data. Right now there is long longitudinal TIR data and that is a problem that they are working to fix. We don't necessarily need to prove long term effects. We know that insulin treats diabetes better than metformin (just with possibly more dangerous side effects - at least with subcutaneous). We know keeping blood sugar in range is better, even for short term. We know managing the disease can prevent it from progressing. We know that usually metformin leads to insulin. What we'd be testing is if metformin actually does what it's supposed to do, and how it compares to what insulin does. It doesn't have to be long term to see if metformin actually keeps A1C low, or if it swings all over the place, yet averages in a good range. |
|
|
|
Post by sayhey24 on Oct 4, 2022 14:49:05 GMT -5
I think Harry is right, afrezza would be the winner by far with proper dosing and who knows maybe some would even see a regression of their diabetes. You would think between this and Affinity 2 Mike could do something www.ncbi.nlm.nih.gov/pmc/articles/PMC4634344/Aged - while the question in the study is should bolus insulin be used as the first step the answer is clearly yes when afrezza is not considered. We have know this for years. How many early insulin studies have been done? So why isn't it - hypos and needles which the industry turned into the two big talking points for the use of antiglycemics. Should RAA's be used before a basal - absolutely. The first thing the PWD loses is post prandial control. Why aren't they - more shots and more chance of hypos and we don't want to mess with the $40B industry. Now with afrezza in the equation its hands down the winner and should be step 1. The problem is afrezza will totally disrupt this industry and every BP will do what they can to stop it. Using afrezza as Step 1 is what VDex does in their SoC and we see their results. Afrezza as step 1 is not a clinical question its sadly all about the money with BP. |
|
|
|
Post by Thundersnow on Oct 4, 2022 16:32:27 GMT -5
I think MNKD (and/or their Partner) will eventually do a massive Afrezza Trial to confirm SUPERIORITY. They will not do it now because they need to use their resources wisely. They will have a steady stream of cashflow for the foreseeable future but it's not enough to fund their pipeline, new R&D and M&A. I also don't think it will happen until AFTER the PEDS Trial is approved and it will give MNKD leverage in negotiating with a new partner. In those negotiations MNKD will have them fund the Superiority Trial because they will have deep pockets. |
|
|
|
Post by prcgorman2 on Oct 4, 2022 16:45:02 GMT -5
I think Harry is right, afrezza would be the winner by far with proper dosing and who knows maybe some would even see a regression of their diabetes. You would think between this and Affinity 2 Mike could do something www.ncbi.nlm.nih.gov/pmc/articles/PMC4634344/Aged - while the question in the study is should bolus insulin be used as the first step the answer is clearly yes when afrezza is not considered. We have know this for years. How many early insulin studies have been done? So why isn't it - hypos and needles which the industry turned into the two big talking points for the use of antiglycemics. Should RAA's be used before a basal - absolutely. The first thing the PWD loses is post prandial control. Why aren't they - more shots and more chance of hypos and we don't want to mess with the $40B industry. Now with afrezza in the equation its hands down the winner and should be step 1. The problem is afrezza will totally disrupt this industry and every BP will do what they can to stop it. Using afrezza as Step 1 is what VDex does in their SoC and we see their results. Afrezza as step 1 is not a clinical question its sadly all about the money with BP. I respectfully question whether the big 3 have any material concern about Afrezza being disruptive. Why should they? Other than perhaps shorting MNKD (which I doubt), I expect they ignore Mannkind, because they can. Mannkind has had bupkis working capital with which to be disruptive starting immediately after Al Mann died in early 2015. It's been an amazing (almost "epic") uphill battle to get to where Mannkind can finally see light (aka "profit") at the end of the long dark tunnel which briefly looked like it led to oblivion.
I recall reading a post recently where it was asserted some pretty serious debt will be maturing in 2025, and revenue may not be sufficient to retire that debt, and interest rates aren't moving in the right direction to be confident the debt can be refinanced on better terms even if Mannkind is in a better financial position than they were during the last refinance. One of the things I like about the current Mannkind leadership style is erring on the side of being conservative even if there are a few minor (and one not-so-minor) examples to the contrary. Interest rates are not yet awful, and perhaps the 3rd quarter results will give reason for optimism such that a capital raise (if needed) or adequate revenue increases result in more aggressive study and marketing action sooner. I'm all for it as soon as Mannkind is.
|
|
|
|
Post by agedhippie on Oct 4, 2022 18:24:32 GMT -5
I think Harry is right, afrezza would be the winner by far with proper dosing and who knows maybe some would even see a regression of their diabetes. You would think between this and Affinity 2 Mike could do something www.ncbi.nlm.nih.gov/pmc/articles/PMC4634344/Aged - while the question in the study is should bolus insulin be used as the first step the answer is clearly yes when afrezza is not considered. We have know this for years. How many early insulin studies have been done? So why isn't it - hypos and needles which the industry turned into the two big talking points for the use of antiglycemics. Should RAA's be used before a basal - absolutely. The first thing the PWD loses is post prandial control. Why aren't they - more shots and more chance of hypos and we don't want to mess with the $40B industry. Now with afrezza in the equation its hands down the winner and should be step 1. The problem is afrezza will totally disrupt this industry and every BP will do what they can to stop it. Using afrezza as Step 1 is what VDex does in their SoC and we see their results. Afrezza as step 1 is not a clinical question its sadly all about the money with BP. There have been no early insulin trials of any size against GLP-1/SGLT2/GIP and those are the current preferred treatments because there is trial data showing outcomes. The early insulin landscape is a mess and lacks a large scale modern trial focused on bolus insulin rather than basal as so many early insulin trials are. I disagree about the early use of bolus vs. basal. Insulin is fungible so if I use basal that now covers my basal needs and restores post prandial control. This is the basis of the early insulin basal trials where they show this. You are always better off using your own insulin for meal times rather than basal. Afrezza could be a hands down winner for step one, but without a trial of the scale Harry gave it remains a pipe dream. There is a difference between opinions and evidence, and trial data is the evidence. Large scale trial or don't be surprised when nothing changes. Personally given the choice between GLP-1 and Afrezza I would go with Afrezza because I don't like GLP-1. However, that would require me to both have a firm opinion on the matter and be prepared to argue with my endo (both of which I do) and most people appear just go with the medical opinion - certainly I do in areas other than diabetes so I can't criticize! I also agree with prcgorman, after 7 years BP don't care about Afrezza in the slightest. They see it as a niche player and they are after the bigger market. In some ways that is not bad as a decent niche can be pretty comfortable. |
|