|
|
Post by uvula on Nov 12, 2022 9:29:54 GMT -5
If mango's comment is correct, how do we turn things around? The cgm companies are partnering with the pump companies. Mnkd can't get the cgm companies to partner with mnkd because the cgm isn't really needed with afrezza once the patient is dialed in.
Is our best hope to get BP to enter a bidding war to buy afrezza? BP was hoping mnkd would die but that is not going to happen.
|
|
|
|
Post by agedhippie on Nov 12, 2022 10:10:19 GMT -5
... This is what we are seeing with the current ADA SoC and A1c. The current theories of treating diabetes are being threatened by Afrezza and MannKind. Afrezza is the only insulin today that matches physiologic insulin and you don’t hear a damn thing about it from ADA. ... The problem with this argument is that Afrezza lacks outcomes data so the benefit cannot be quantified. Until there is outcomes data this will not change. This is the point that Stevil and I are making - we both think Afrezza is a better insulin, but we also know that until it can be shown that this materially impacts outcomes based on specific trial data change will be slow (glacial?) This is the reason UTHR ran the TETON trial to get that data for Tyvaso for PAH-ILD, and that Janssen ran CREDENCE to prove that SGLT2 reduced CKD despite being a diabetes drug. In both cases there was pretty solid reasoning for thinking these worked, but until there those trials they were marginal in those roles because medicine requires proof and proof is trial data. |
|
|
|
Post by agedhippie on Nov 12, 2022 10:31:51 GMT -5
If mango's comment is correct, how do we turn things around? The cgm companies are partnering with the pump companies. Mnkd can't get the cgm companies to partner with mnkd because the cgm isn't really needed with afrezza once the patient is dialed in. Is our best hope to get BP to enter a bidding war to buy afrezza? BP was hoping mnkd would die but that is not going to happen. CGM makers partnering with pumps is because the CGM is an integral component of automated delivery (AID). Pumps sell CGMs. The CGM makers are not blind though which is why they are spending a ton of money on developing the Type 2 market who for the most part will never be on a pump. |
|
|
|
Post by phdedieu12 on Nov 12, 2022 10:47:32 GMT -5
If mango's comment is correct, how do we turn things around? The cgm companies are partnering with the pump companies. Mnkd can't get the cgm companies to partner with mnkd because the cgm isn't really needed with afrezza once the patient is dialed in. Is our best hope to get BP to enter a bidding war to buy afrezza? BP was hoping mnkd would die but that is not going to happen. There is no such thing as turning things around. The largest pharma companies couldn't sell inhaled insulin on a big scale (Pfizer failed with Exubera, and we all know what Sanofi did), and the reality is that Afrezza will be a niche product for the foreseeable future with a rather slow growth, and what will likely accelerate the adoption is good results with peds. It's a smaller subset of endos, easier to target and educate, and those patients continue as adults bringing Afrezza along. |
|
|
|
Post by mango on Nov 12, 2022 11:08:19 GMT -5
... This is what we are seeing with the current ADA SoC and A1c. The current theories of treating diabetes are being threatened by Afrezza and MannKind. Afrezza is the only insulin today that matches physiologic insulin and you don’t hear a damn thing about it from ADA. ... The problem with this argument is that Afrezza lacks outcomes data so the benefit cannot be quantified. Until there is outcomes data this will not change. This is the point that Stevil and I are making - we both think Afrezza is a better insulin, but we also know that until it can be shown that this materially impacts outcomes based on specific trial data change will be slow (glacial?) This is the reason UTHR ran the TETON trial to get that data for Tyvaso for PAH-ILD, and that Janssen ran CREDENCE to prove that SGLT2 reduced CKD despite being a diabetes drug. In both cases there was pretty solid reasoning for thinking these worked, but until there those trials they were marginal in those roles because medicine requires proof and proof is trial data. We already know diabetes is just the dysregulation of blood glucose. Nothing more, nothing less. Afrezza fixes the underlying issue of the loss of first phase insulin, which is directly responsible for the dysregulation (regardless of what caused the loss of the first phase!). The unique thing about Afrezza is it’s just human insulin and behaves exactly like it. We already have all the information we need. The difference between Afrezza and everything else is Afrezza replaces what is missing while everything else in the ADA’s SoC simply puts a bandaid on symptoms, while causes new problems—they do nothing to address the first phase which is the underlying issue that has been proved to restore glucose homeostasis when it is brought back in the picture (via Afrezza which is just the same insulin the body already has). We don’t need redundant studies when we already have the quantifiable information already. |
|
|
|
Post by mango on Nov 12, 2022 11:22:40 GMT -5
If mango's comment is correct, how do we turn things around? The cgm companies are partnering with the pump companies. Mnkd can't get the cgm companies to partner with mnkd because the cgm isn't really needed with afrezza once the patient is dialed in. Is our best hope to get BP to enter a bidding war to buy afrezza? BP was hoping mnkd would die but that is not going to happen. It’ll likely never change. People say they want to see studies proving glucose homeostasis is good for our health. We already have that information. Why do we need new Afrezza studies showing us things we already know? Afrezza is just human insulin. It’s identical to what’s in the human body and behaves exactly like it as well. All Afrezza does is restores something in the human body that is missing. Once that happens, glucose homeostasis returns and we already know why glucose homeostasis is vital for our health and the consequences of a dysregulated one (see all the diabetic related health complications!). |
|
|
|
Post by agedhippie on Nov 12, 2022 16:18:55 GMT -5
There is no such thing as turning things around. The largest pharma companies couldn't sell inhaled insulin on a big scale (Pfizer failed with Exubera, and we all know what Sanofi did), and the reality is that Afrezza will be a niche product for the foreseeable future with a rather slow growth, and what will likely accelerate the adoption is good results with peds. It's a smaller subset of endos, easier to target and educate, and those patients continue as adults bringing Afrezza along. Although it pains me to agree with phdedieu,  , he is right. Adoption in the peds market will drive the adult market, but the adult market will be slow because there are typically different endos for kids and adults. The interesting bit will be when those kids transition from the kids to adults endos and insist on bringing Afrezza along - that will force a reevaluation on the adult side. Getting a new diabetic onboard is always going to be easier than converting existing diabetics in the Type 1 world. |
|
|
|
Post by agedhippie on Nov 12, 2022 16:52:02 GMT -5
It’ll likely never change. People say they want to see studies proving glucose homeostasis is good for our health. We already have that information. Why do we need new Afrezza studies showing us things we already know? Afrezza is just human insulin. It’s identical to what’s in the human body and behaves exactly like it as well. All Afrezza does is restores something in the human body that is missing. Once that happens, glucose homeostasis returns and we already know why glucose homeostasis is vital for our health and the consequences of a dysregulated one (see all the diabetic related health complications!). We don't need studies to prove that glucose homeostasis is good, everyone agree on that. What we do need is trials to support the claim that Afrezza causes glucose homeostasis to return - it puts Type 2 into remission. That data doesn't exist in meaningful trial data, and just saying it's obvious isn't going to cut it. The medical world will want to see this happen in a trial setting, and to see if the effect persists or trails off over time. By all means ignore the established process (clinical trials) and don't do the trials, but in that case expect *very* slow progress in the Type 2 market. |
|
|
|
Post by sayhey24 on Nov 13, 2022 8:46:39 GMT -5
It’ll likely never change. People say they want to see studies proving glucose homeostasis is good for our health. We already have that information. Why do we need new Afrezza studies showing us things we already know? Afrezza is just human insulin. It’s identical to what’s in the human body and behaves exactly like it as well. All Afrezza does is restores something in the human body that is missing. Once that happens, glucose homeostasis returns and we already know why glucose homeostasis is vital for our health and the consequences of a dysregulated one (see all the diabetic related health complications!). We don't need studies to prove that glucose homeostasis is good, everyone agree on that. What we do need is trials to support the claim that Afrezza causes glucose homeostasis to return - it puts Type 2 into remission. That data doesn't exist in meaningful trial data, and just saying it's obvious isn't going to cut it. The medical world will want to see this happen in a trial setting, and to see if the effect persists or trails off over time. By all means ignore the established process (clinical trials) and don't do the trials, but in that case expect *very* slow progress in the Type 2 market. If I remember correctly in some of Kendall's nuggets of gold, use of afrezza showed regression. In the short term this was demonstrated by needing less afrezza once they got the T2 PWDs within a near normal range and "resistance" was reduced. The thing is selling afrezza is not about studies. We have the ABC study and the 175 and Kendall's nuggets. We should refactor the ABC with SGLT2 and Mounjaro. In fact the more studies with afrezza the better because we already know the answer - afrezza WINS! Nothing is better than a healthy pancreas and afrezza is pretty damn good at replicating post prandial control. What long term studies did Merck have when Januvia hit the market in 2006? Lilly was just starting to get things in place for Exubera and the next thing you know Januvia is selling 40,000 scripts a week. Why? It was surely not any studies or trials. How many people have died since 2006 from fatal hemorrhagic and necrotizing pancreatitis? How many have failed on the "Step Program" using Januvia? To address your comment above - "CGM makers partnering with pumps is because the CGM is an integral component of automated delivery (AID). Pumps sell CGMs. The CGM makers are not blind though which is why they are spending a ton of money on developing the Type 2 market who for the most part will never be on a pump." Here is what Mike said on the call - "In 2023, we fully expect that Afrezza will be covered in Medicare at $35 under the Inflation Protection Act bill that was passed. That changes the game as one of the major objectives for Afrezza's around access, and we really want to continue to see that patients can only have to pay $35 for Afrezza" For the CGM makers who want to develop that HUGE T2 market - Januvia, the SGLT2s and GLP1s can NOT help sell CGMs but people using insulin can. If a PWD is using insulin - inhaled included - Medicare will pay for the CGM. I am sure that has Kevin Sayer's attention. While as a purest I would like to see afrezza as step 1 targeting the 45-64 age range getting the sales at 65+ will be great. Lets see if Mike is correct and this "changes the game". I think getting an Abbott and their money as an advocate can only help. |
|
|
|
Post by agedhippie on Nov 13, 2022 10:28:53 GMT -5
If I remember correctly in some of Kendall's nuggets of gold, use of afrezza showed regression. In the short term this was demonstrated by needing less afrezza once they got the T2 PWDs within a near normal range and "resistance" was reduced. The thing is selling afrezza is not about studies. We have the ABC study and the 175 and Kendall's nuggets. We should refactor the ABC with SGLT2 and Mounjaro. In fact the more studies with afrezza the better because we already know the answer - afrezza WINS! Nothing is better than a healthy pancreas and afrezza is pretty damn good at replicating post prandial control. What long term studies did Merck have when Januvia hit the market in 2006? Lilly was just starting to get things in place for Exubera and the next thing you know Januvia is selling 40,000 scripts a week. Why? It was surely not any studies or trials. How many people have died since 2006 from fatal hemorrhagic and necrotizing pancreatitis? How many have failed on the "Step Program" using Januvia? To address your comment above - "CGM makers partnering with pumps is because the CGM is an integral component of automated delivery (AID). Pumps sell CGMs. The CGM makers are not blind though which is why they are spending a ton of money on developing the Type 2 market who for the most part will never be on a pump." Here is what Mike said on the call - "In 2023, we fully expect that Afrezza will be covered in Medicare at $35 under the Inflation Protection Act bill that was passed. That changes the game as one of the major objectives for Afrezza's around access, and we really want to continue to see that patients can only have to pay $35 for Afrezza" ... There is science to support the idea that insulin at least partially restores the beta cells after a prolonged period of hypoglycemia - this is know as the honeymoon period and is most clearly seen in new onset Type 1. I would expect the same effect in Type 2 and that this would account for the drop in the need for insulin you see with Afrezza. Why you need studies is to see if that effect persists (the honeymoon period sadly doesn't) and how the insulin need varies over the longer term. Entertainingly the example you use, Januvia, had an even bigger difference between the drug and placebo than Afrezza did in the same 175 trial setup (phase 3). This is why nobody cares about 175, if you can't beat the placebo the drug is not getting launched - it's a pass/fail exam. The ABC study was interesting in so far as it goes, but the very limit numbers in an arm (eight!) mean that it's always going to viewed as suspect. As to the 60 minute number, that was already know from STAT, remember this chart? You can see why the PR pushed the 60 minute number  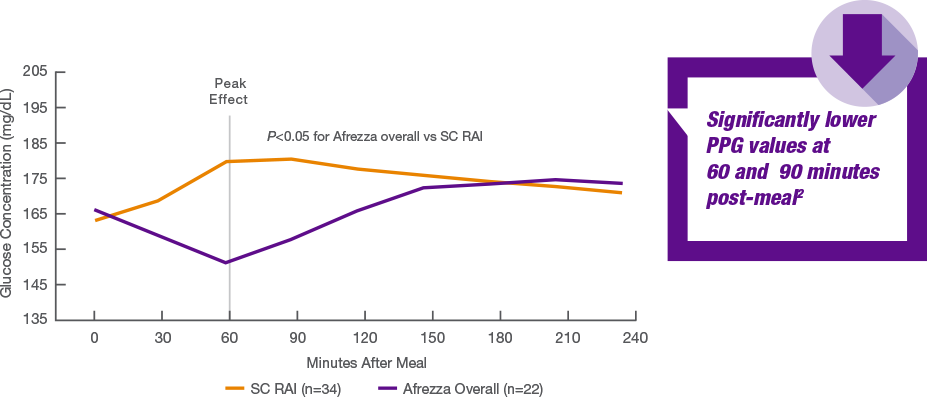 As to Januvia; Merck did no long term studies before launch, nobody does those pre-launch. You push the drug out as soon as you have phase 3 and approval. Long term studies by definition have to be done after launch. In fairness I wouldn't touch DPP-4. I am curious if you will be able to get Afrezza if it's not in your Medicare D insurer's formulary. Some insurers will cover non-preferred drugs with a worse co-pay and the $35 limit may apply, others won't cover off-formulary drugs at all. |
|
|
|
Post by agedhippie on Nov 13, 2022 10:36:51 GMT -5
... I am curious if you will be able to get Afrezza if it's not in your Medicare D insurer's formulary. Some insurers will cover non-preferred drugs with a worse co-pay and the $35 limit may apply, others won't cover off-formulary drugs at all. I can answer my own question! Under the new Inflation Reduction Act requirement, Part D plans are not required to cover all available insulin products at the $35 monthly copayment amount, only those insulin products that are covered on a plan’s formulary.
|
|
|
|
Post by sayhey24 on Nov 13, 2022 18:11:20 GMT -5
Aged - have you ever tried to understand Medicare Advantage Plans? Most assume Part D coverage provided by Uncle Sam but not all? They account for about 48% of Part D recipients.
For 2023 no one is covering afrezza. Once Uncle Sam's Part D covers it then "Some" Advantage plans will cover afrezza but each recipient will need to pick the proper plan. Each Advantage plan is different but nearly the same. It nearly requires a Phd to navigate the plans but when afrezza becomes available people will find it because they will be specifically looking for it. They can then switch during open enrollment which runs mid October through early December. Coverage would then start 1/1/2024. Plans will offer afrezza to attract recipients as its a highly competitive market and Uncle Sam is reimbursing anyway. Additionally about 52% have a traditional part D plan which will get coverage if Mike is correct in 2023 but more likely 1/1/2024.
Concerning the honeymoon period this is a T1 thing and not all T1s. It really depends on how much beta cell mass you have left. If you basically have none then you will probably have the honeymoon period. If you have none then you have had no cytokine activity messing with the receptors. Over time most build "some" resistance but nothing like a T2 because pancreatic release is limited. In the T2 the pancreas has been working overtime releasing its junk.
In T2s it actually works in reverse. You have extreme resistance at first but as you bring the PWD back into range and take the load off the pancreas so it does not need to release its "insulin", resistance will reduce. It goes back to the Wensveen theory. What is being released by the pancreas is causing the resistance. Figure 2 outlines the activity ncbi.nlm.nih.gov/pmc/articles/PMC8050380/
|
|
|
|
Post by sayhey24 on Nov 13, 2022 18:32:24 GMT -5
Aged - BTW if afrezza had been properly dosed it would have kicked Januvia's butt in the 175. Here we have the 175 study which showed superiority and afrezza was grossly under dosed. I would be all for adding Januvia along with the SGLT2 and GLP1s to a refactoring of the ABC study. I am sure afrezza will WIN!
|
|
|
|
Post by Thundersnow on Nov 13, 2022 19:13:31 GMT -5
Aged - BTW if afrezza had been properly dosed it would have kicked Januvia's butt in the 175. Here we have the 175 study which showed superiority and afrezza was grossly under dosed. I would be all for adding Januvia along with the SGLT2 and GLP1s to a refactoring of the ABC study. I am sure afrezza will WIN! And you're not blaming Al Mann??? You blame Pfizer and Sanofi but you don't blame MNKD nor AL for not going the extra step(s) to make sure Afrezza would succeed against its competitors. I know he spent a ton of money plus the 2 CRLS and we all know he just wanted to get Afrezza approved. Well that will cost Afrezza a DECADE...... But its good for us LONGS bc we were able to add MNKD at lower prices!! |
|
|
|
Post by sayhey24 on Nov 14, 2022 8:15:23 GMT -5
Aged - BTW if afrezza had been properly dosed it would have kicked Januvia's butt in the 175. Here we have the 175 study which showed superiority and afrezza was grossly under dosed. I would be all for adding Januvia along with the SGLT2 and GLP1s to a refactoring of the ABC study. I am sure afrezza will WIN! And you're not blaming Al Mann??? You blame Pfizer and Sanofi but you don't blame MNKD nor AL for not going the extra step(s) to make sure Afrezza would succeed against its competitors. I know he spent a ton of money plus the 2 CRLS and we all know he just wanted to get Afrezza approved. Well that will cost Afrezza a DECADE...... But its good for us LONGS bc we were able to add MNKD at lower prices!! I think if you follow my posts I am like a broken record saying the same thing over and over. What I have said many times is the biggest marketing mistake MNKD ever made was calling the cartridges "units". They should have been called small, med. and large. That is squarely on Al Mann. I understand why he did and at the time it seemed to make sense but hopefully Mike will change that with new packaging and a label change. Or, come out with a product for T1s and a separate one for T2s which might make more sense. As far as Pfizer and Sanofi I will go even further and point the finger at Brandicourt. Exubera was his baby at Pfizer and between afrezza and Januvia it went bust. Then he shows up at Sanofi and deep sixes afrezza day 1. Talk about MNKD being snake bit. As far as what MNKD got from the 175 study was superiority and a 14-0 vote at Adcom to approve. That was pretty damn good. Sanofi was then suppose to do phase 4 studies. Can you point me to one? Lets remember the big fear of insulin - hypoglycemia. During the 175 study to get approval if you can get superiority and still be conservative so not risking severe hypos that seems like the prudent thing to do. The phase 4 studies should then show the super-duper superiority. The problem now is the approved label needs updating. When was the last time it was updated? I think it would have better for us longs if afrezza realized Al Mann's belief five years ago and became the greatest selling drug of all time. There would not have been a split and the pps would be 100+. There would also be a lot of PWDs who would have already realized life changing care. |
|