|
|
Post by agedhippie on Nov 15, 2022 10:02:31 GMT -5
Ha! Aged keeps saying that. The placebo was metformin alone or metformin plus 1 or 2 additional oral agents plus technosphere powder with no insulin. Oral agents included things like Januvia. Its pretty simple nothing is going to be better than pancreatic insulin post meal which it basically what we have with afrezza. The 175 trial was a good trial with the exception that afrezza was under dose. The good news is while it showed superiority we know it would have done even better if afrezza was properly dose. In summary for those that don't want to read all the gory details. The trial requirement were that you were at the maximum dose for the oral drugs you were taking and that you still had an HbA1c over 7.5. Neither arm discontinued the drugs they were taking at the start so the Afrezza arm continued to take metformin, and any other oral meds they were on at the start. The only difference was that half the people were using Afrezza and the other half were using pure Technosphere (that was the placebo). This is the standard Phase 3 protocol for all clinical drug trials - drug against placebo. All drugs that pass phase 3 are superior by definition! Now the gory details... How do we know this? Well, read the official document! Here it is: Comparison of Technosphere® Insulin Versus Technosphere Powder (Placebo) in Insulin-Naive Subjects With Type 2 Diabetes Mellitus. Note - insulin vs. placebo, not insulin vs. oral meds. But surely they won't remain on oral meds if they are on Afrezza? Lets look at the official trial description: Insulin-naive subjects with Type 2 Diabetes Mellitus who are sub-optimally controlled on either maximum tolerated dose of metformin or maximum tolerated dose of metformin plus one or two other oral anti-diabetic medications will have either Prandial Technosphere® Insulin or Technosphere Powder (placebo) added to their oral antidiabetic drugs.What is meant by maximum dose, won't the placebo arm just increase their dose? No, they cannot, they are already taking as much as they can take safely. Lets check the official inclusion criteria: Currently receiving as diabetes treatment only metformin or 2 or more OADs and on stable doses for at least 3 months before enrollment
- Subjects receiving metformin must be on at least 1.5gm daily, or up to the maximum tolerated dose
- Subjects treated with a sulfonylurea must be on at least 50% of the total maximum approved dose for a given agent
- Subjects receiving a DPP-4 inhibitor must receive the maximum approved dose specific for that agent
- Metiglinide and alpha-glucoside inhibitors must be taken at the highest tolerated dose within the approved dose rangeIn future when the 175 trial comes up I am going to link back to this post as an explanation of why the endos do not care. Of course Afrezza can outperform a placebo, it would be news if it couldn't. |
|
|
|
Post by sayhey24 on Nov 15, 2022 16:36:45 GMT -5
Aged - I am not sure if we are in violent agreement or not. The 175 followed the ADA step program and was a great study. Thats the protocol the FDA wanted because it matches the ADA's SoC. This is how they treat T2s. Do you want something else?
As a T2 you get metformin first. Then when that fails you get something else with the metformin. Lets say you and I both got Januvia. So we are both on metformin and Januvia. Now thats failing so we both got afrezza as part of the trial except I got afrezza in my cartridge and you only got technosphere with no insulin aka "placebo".
Of course I win because I got afrezza. The thing is afrezza when properly dose will always win because it mimics a healthy pancreas. Even in this case when underdosed it won. A healthy pancreas will always beat all of this junk. We don't need a 10 year study to tell us that. We also have shown the afrezza pk profile mimics a healthy pancreas for post prandial control. The thing is afrezza should be step 1 and not an add on and will never need an oral agent to be added to it.
What is it you don't like? Is it the ADA step program? I agree its terrible but as long as we keep giving these oral agents thats as good as we can do. They will fail and what the ADA has is a treat to fail protocol. In my example, did you want to take us both off Januvia? We can not do that because we don't know who is getting the "placebo" cartridge and we are both already failing.
|
|
|
|
Post by oldfishtowner on Nov 16, 2022 9:16:18 GMT -5
Aged - I am not sure if we are in violent agreement or not. The 175 followed the ADA step program and was a great study. Thats the protocol the FDA wanted because it matches the ADA's SoC. This is how they treat T2s. Do you want something else? As a T2 you get metformin first. Then when that fails you get something else with the metformin. Lets say you and I both got Januvia. So we are both on metformin and Januvia. Now thats failing so we both got afrezza as part of the trial except I got afrezza in my cartridge and you only got technosphere with no insulin aka "placebo". Of course I win because I got afrezza. The thing is afrezza when properly dose will always win because it mimics a healthy pancreas. Even in this case when under dosed it won. A healthy pancreas will always beat all of this junk. We don't need a 10 year study to tell us that. We also have shown the afrezza pk profile mimics a healthy pancreas for post prandial control. The thing is afrezza should be step 1 and not an add on and will never need an oral agent to be added to it. What is it you don't like? Is it the ADA step program? I agree its terrible but as long as we keep giving these oral agents thats as good as we can do. They will fail and what the ADA has is a treat to fail protocol. In my example, did you want to take us both off Januvia? We can not do that because we don't know who is getting the "placebo" cartridge and we are both already failing. Yes, we do not like the ADA step program as it now exists in the SOC today. Because, yes Afrezza should be step one and would probably be the only step necessary if taken early enough and properly dosed.
And so isn't the answer a head-to-head phase 3 trial against metformin, and against januvia and whatever else is necessary to make Afrezza the preferred and only choice for T2? And what do we have, the same old same old with Cipla's trial - not head to head with metformin, but as an add-on. Why? As you have said, it has already been done, maybe not with the higher dose, but so what.
|
|
|
|
Post by sayhey24 on Nov 16, 2022 14:49:11 GMT -5
The head to head is basically what you got in the 175. If you and I were on metformin and then I got afrezza there is the head to head. If you want to take me off the metformin thats outside the ADA SoC guidelines. The SoC is additive.
Could we take me off metformin and see how I do on afrezza alone, sure. We already know the answer, afrezza wins. We could do the same with Januvia and Ozempic and Mounjaro - put me on it then take me off and switch to afrezza, afrezza will win again. This I think is what Aged and myself have suggested for mounjaro. Who knows maybe Mike is thinking about it too.
The thing is afrezza will always win or at least be as good without all the potential side effects. With Mounjaro they will win on weight loss and thats fine unless of course when on afrezza you are limited to what you ate on mounjaro. If the goal is weight loss take the Mounjaro. If its BG control take the afrezza.
None of these oral agents will beat a healthy pancreas and afrezza mimics the pancreas with the same insulin. Mike already has a superiority study with the 175 and India will again show superiority. Why are we doing the 175 in India - they are just trying to get India to approve it. At this point Mike has all the pieces to put a T2 plan together. His biggest problem is probably that he does not have enough clout to make it happen and he doesn't want to risk the money. Without a well funded partner his approach is slow and steady. With the kids study afrezza will win so adoption in the T1 space will grow. Maybe we get the URAA class and he can leverage that both with T1 and T2s. Maybe India helps with manufacturing overhead costs. Mike thinks medicare will cover in 2023. When that happens maybe a DXCM and/or Abbott show a big interest since afrezza can help sell CGMs in the T2 space.
|
|
|
|
Post by nylefty on Nov 17, 2022 1:52:18 GMT -5
Mike thinks medicare will cover in 2023. When that happens maybe a DXCM and/or Abbott show a big interest since afrezza can help sell CGMs in the T2 space. It's up to the insurance companies as to whether they cover Afrezza when it comes to Medicare patients. They would make the decision, not "Medicare." |
|
|
|
Post by sayhey24 on Nov 17, 2022 7:24:27 GMT -5
Mike thinks medicare will cover in 2023. When that happens maybe a DXCM and/or Abbott show a big interest since afrezza can help sell CGMs in the T2 space. It's up to the insurance companies as to whether they cover Afrezza when it comes to Medicare patients. They would make the decision, not "Medicare." This was not me saying this - it was Mike. I am assuming he knows something or he would not have made that statement. He talked about the URAA class. Maybe thats how he thinks its happening as "inhaled" insulin. My guess is they got the word "inhaled" as a "Part D form". If so ALL Part D plans are required to cover it. If so Mike is correct - its a game changer. "Under the current Part D insulin model, participating plans are not required to cover all insulin products at the $35 monthly copayment amount, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting). Absent a requirement to cover all insulin products at no more than a $35 copay, insulin users might need to switch from one insulin product to another to save on their out-of-pocket costs, or switch to a plan that covers their insulin product at the $35 copayment." www.kff.org/medicare/issue-brief/insulin-out-of-pocket-costs-in-medicare-part-d/#:~:text=Under%20the%20current%20Part%20D,%2C%20and%20long%2Dacting). |
|
|
|
Post by liane on Nov 17, 2022 7:55:38 GMT -5
Regarding Part D:
A drug category is a group of drugs that treat the same symptoms or have similar effects on the body. All Part D plans must include at least two drugs from most categories and must cover all drugs available in the following categories:
HIV/AIDS treatments
Antidepressants
Antipsychotic medications
Anticonvulsive treatments for seizure disorders
Immunosuppressant drugs
Anticancer drugs (unless covered by Part B)
So maybe for URAA TI fits the bill?
|
|
|
|
Post by prcgorman2 on Nov 17, 2022 8:15:09 GMT -5
That would be excellent. Is there a Medicare formulary that will show that? If so, when? (I’ll research the answers too, but just putting it out there.)
|
|
|
|
Post by peppy on Nov 17, 2022 8:17:15 GMT -5
Regarding Part D:
A drug category is a group of drugs that treat the same symptoms or have similar effects on the body. All Part D plans must include at least two drugs from most categories and must cover all drugs available in the following categories:
HIV/AIDS treatments
Antidepressants
Antipsychotic medications
Anticonvulsive treatments for seizure disorders
Immunosuppressant drugs
Anticancer drugs (unless covered by Part B)
So maybe for URAA TI fits the bill?
 Catch 22 |
|
|
|
Post by oldfishtowner on Nov 17, 2022 8:17:41 GMT -5
The head to head is basically what you got in the 175. If you and I were on metformin and then I got afrezza there is the head to head. If you want to take me off the metformin thats outside the ADA SoC guidelines. The SoC is additive. Could we take me off metformin and see how I do on afrezza alone, sure. We already know the answer, afrezza wins. We could do the same with Januvia and Ozempic and Mounjaro - put me on it then take me off and switch to afrezza, afrezza will win again. This I think is what Aged and myself have suggested for mounjaro. Who knows maybe Mike is thinking about it too. The thing is afrezza will always win or at least be as good without all the potential side effects. With Mounjaro they will win on weight loss and thats fine unless of course when on afrezza you are limited to what you ate on mounjaro. If the goal is weight loss take the Mounjaro. If its BG control take the afrezza. None of these oral agents will beat a healthy pancreas and afrezza mimics the pancreas with the same insulin. Mike already has a superiority study with the 175 and India will again show superiority. Why are we doing the 175 in India - they are just trying to get India to approve it. At this point Mike has all the pieces to put a T2 plan together. His biggest problem is probably that he does not have enough clout to make it happen and he doesn't want to risk the money. Without a well funded partner his approach is slow and steady. With the kids study afrezza will win so adoption in the T1 space will grow. Maybe we get the URAA class and he can leverage that both with T1 and T2s. Maybe India helps with manufacturing overhead costs. Mike thinks medicare will cover in 2023. When that happens maybe a DXCM and/or Abbott show a big interest since afrezza can help sell CGMs in the T2 space. " If you want to take me off the metformin thats outside the ADA SoC guidelines. The SoC is additive." Why is this pertinent? If you want to change the SOC, don't you need a clinical trial that is outside the current SOC? |
|
|
|
Post by oldfishtowner on Nov 17, 2022 8:21:36 GMT -5
It's up to the insurance companies as to whether they cover Afrezza when it comes to Medicare patients. They would make the decision, not "Medicare." This was not me saying this - it was Mike. I am assuming he knows something or he would not have made that statement. He talked about the URAA class. Maybe thats how he thinks its happening as "inhaled" insulin. My guess is they got the word "inhaled" as a "Part D form". If so ALL Part D plans are required to cover it. If so Mike is correct - its a game changer. "Under the current Part D insulin model, participating plans are not required to cover all insulin products at the $35 monthly copayment amount, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting). Absent a requirement to cover all insulin products at no more than a $35 copay, insulin users might need to switch from one insulin product to another to save on their out-of-pocket costs, or switch to a plan that covers their insulin product at the $35 copayment." www.kff.org/medicare/issue-brief/insulin-out-of-pocket-costs-in-medicare-part-d/#:~:text=Under%20the%20current%20Part%20D,%2C%20and%20long%2Dacting). ""Under the current Part D insulin model, participating plans are not required to cover all insulin products at the $35 monthly copayment amount, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting)."
Vial, pen --- where is inhaled?
|
|
|
|
Post by prcgorman2 on Nov 17, 2022 10:45:28 GMT -5
This was not me saying this - it was Mike. I am assuming he knows something or he would not have made that statement. He talked about the URAA class. Maybe thats how he thinks its happening as "inhaled" insulin. My guess is they got the word "inhaled" as a "Part D form". If so ALL Part D plans are required to cover it. If so Mike is correct - its a game changer. "Under the current Part D insulin model, participating plans are not required to cover all insulin products at the $35 monthly copayment amount, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting). Absent a requirement to cover all insulin products at no more than a $35 copay, insulin users might need to switch from one insulin product to another to save on their out-of-pocket costs, or switch to a plan that covers their insulin product at the $35 copayment." www.kff.org/medicare/issue-brief/insulin-out-of-pocket-costs-in-medicare-part-d/#:~:text=Under%20the%20current%20Part%20D,%2C%20and%20long%2Dacting). ""Under the current Part D insulin model, participating plans are not required to cover all insulin products at the $35 monthly copayment amount, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting)."
Vial, pen --- where is inhaled?
Right next to intravenous.  |
|
|
|
Post by sayhey24 on Nov 17, 2022 14:39:18 GMT -5
Regarding Part D:
A drug category is a group of drugs that treat the same symptoms or have similar effects on the body. All Part D plans must include at least two drugs from most categories and must cover all drugs available in the following categories:
HIV/AIDS treatments
Antidepressants
Antipsychotic medications
Anticonvulsive treatments for seizure disorders
Immunosuppressant drugs
Anticancer drugs (unless covered by Part B)
So maybe for URAA TI fits the bill?
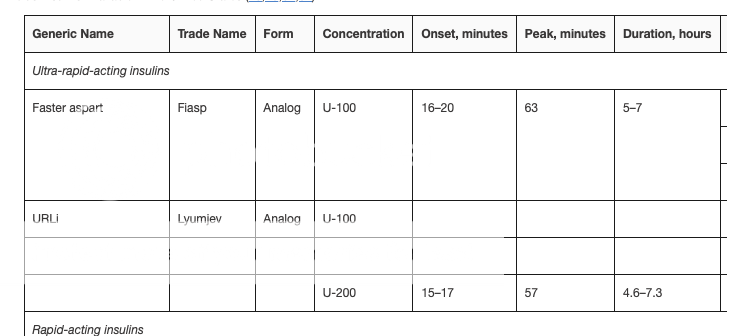
 Catch 22 I think Mike expects it to be approved under the "form" requirement and not the "type". The form would be "inhaled" vs pen or vial. If this is a new form type all Part D plans would be required to include it as there is no other "inhaled" dosage form. We will have to see but he seemed pretty sure it was getting coverage. I guess we should pull the legislation and stop guessing. Peppy - where is the above chart from - Medicare.gov??? The below was Published: Jul 28, 2022 so it should be current and does not include ultra rapid acting. "Under the current Part D insulin model, participating plans are not required to cover all insulin products at the $35 monthly copayment amount, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting). Absent a requirement to cover all insulin products at no more than a $35 copay, insulin users might need to switch from one insulin product to another to save on their out-of-pocket costs, or switch to a plan that covers their insulin product at the $35 copayment." www.kff.org/medicare/issue-brief/insulin-out-of-pocket-costs-in-medicare-part-d/#:~:text=Under%20the%20current%20Part%20D |
|
|
|
Post by mytakeonit on Nov 17, 2022 15:19:05 GMT -5
What insulin is covered by Medicare Part D?
Available to all Medicare beneficiaries, Part D is Medicare’s prescription drug program. You can purchase a drug plan that meets your needs through a private, Medicare-approved insurance carrier. If you have Medicare Advantage (Part C), you may be able to get your drug coverage that way.
Part D covers injectable and inhaled insulin that's not used with an insulin pump. It also covers certain medical supplies used to inject or inhale insulin, like inhaled insulin devices, syringes, gauze, needles, and alcohol swabs.
Most Part D plans charge a monthly premium that’s separate from the Medicare Part B premium you may already be paying. You will be responsible for other out-of-pocket costs, too, such as coinsurance or copayments. Many drug plans also have a deductible that you must satisfy before your plan coverage kicks in.
|
|
|
|
Post by peppy on Nov 17, 2022 15:32:44 GMT -5
What insulin is covered by Medicare Part D? Available to all Medicare beneficiaries, Part D is Medicare’s prescription drug program. You can purchase a drug plan that meets your needs through a private, Medicare-approved insurance carrier. If you have Medicare Advantage (Part C), you may be able to get your drug coverage that way. Part D covers injectable and inhaled insulin that's not used with an insulin pump. It also covers certain medical supplies used to inject or inhale insulin, like inhaled insulin devices, syringes, gauze, needles, and alcohol swabs. Most Part D plans charge a monthly premium that’s separate from the Medicare Part B premium you may already be paying. You will be responsible for other out-of-pocket costs, too, such as coinsurance or copayments. Many drug plans also have a deductible that you must satisfy before your plan coverage kicks in. I bought a cheap Part D. I looked at it. I knew it was cheap, all the plan did was allow the price to be in tiers. Remember Tiers? Afrezza was not listed. ***** Another thing, "just one of each dosage form (vial, pen) and insulin type ( rapid-acting, short-acting, intermediate-acting, and long-acting). " **** No ultra fast acting insulin listed. Catch 44 twice as good as 22. I feel like agedhippie, "Nope" |
|





