|
|
Post by Thundersnow on Dec 15, 2022 9:17:15 GMT -5
Just so we are on the same page we are talking about V-Go. This is the user manual www.go-vgo.com/instructions-for-patient-use/How Mike expects to use this for something else might take some imagination, more imagination than I have. It comes in three fixed sizes 20/30/40. The selling point of this device is it allows the PWD to push the button at mealtime and bolus. This device is only T2 only approved. Which means this is for T2s who have already been put on basal insulin and are still failing the ADA step program and have reached the final step - mealtime insulin. IMO, to repurpose this device you need an application which needs a predetermined steady state release but also requires bolusing from time to time which is going to be done manually by pushing the button. I guess there could be another application but aside from diabetes I am not sure what. This device would also require re-engineering if it needed to deliver something other than the pre-configured 20/30/40 units. Does UTHR have a drug which fits the V-Go better than an electric pump, IDK. What I do know is the relationship Martine had with Al Mann started when he made a Minimed pump for her kid. Its possible UTHR might have something to fit the V-Go but I would be surprised. In my day I have bought and sold a few companies. I have also gotten up at the closing of several companies and walked away from the table. These acquisitions would take months and months. It can get pretty exciting during the process - like buying your first house. I think thats what we have with V-Go but I know you and others have a different opinion which is good. What I do know is that we will be in about $20M by year end. I also know that right now its like the old "I Love Lucy" show where she was bottling beer and Rickie asked her how much she was losing per bottle and she told him 10cents but she was going to make up the loss in volume. I also know the selling point of this device is to avoid MDI for the T2. Its to allow the T2 to push the button at mealtime and avoid the injections. Do you see a problem here with afrezza? The point of afrezza is to avoid the injection, avoid the hypo from the RAA and provide the PWD with significantly better mealtime control. Now we are going to put this in the "sales reps bag" in January and when they can't explain why afrezza is better we are gong to say forget it - just use this device with your favorite RAA. Thank you sayhey24 I always enjoy reading your viewpoints and thoughts. You and I are equally and genuinely invested into this story. While you make great points, I can't help but be intrigued by what MC means when he says "...re purpose outside diabetes." Just exactly what he has in mind is unknown but like I said, I'll wait before making judgement calls. Talking about imagination, 50 years ago who would've thought you could fit that big heavy box on the living room floor broadcasting only three networks with those telescopic antennas into that tiny little phone hanging over on that wall in the kitchen? Not me. It could've been because I was watching too much Happy Days keeping me stuck in the 50's. I enjoy your opinions but it's obvious SAYHEY is a GLASS HALF EMPTY GUY. He has NO VISION when it comes to developing A PLATFORM Device. Let's wait and see what Mike has up his sleeve. So far Mike has don't a decent job as CEO with the MC going from $150M to $1.2B. V-Go Revenues last Qtr were $5.4M which is damn good for a product that was virtually written off. Let's wait and see. |
|
|
|
Post by sayhey24 on Dec 15, 2022 13:46:33 GMT -5
Thundersnow - How much money did MNKD make or lose with V-Go last quarter? Was it a net loser and will it be a loser next quarter? How does a sales rep try and sell afrezza as the replacement to RAAs and then turn around and say I got a device here which will work with your RAA? What do the reps say - the RAAs are better than afrezza??? You can't have it both ways. They should be converting any doctor prescribing V-Go to afrezza. That $5M should be afrezza sales and they should be filling the dumpster with V-Gos. These should be easy targets. They know the doctors, they know V-Go and they should be able to easily explain why afrezza is far superior.
I don't want a half full or half empty glass. I want a full glass. I am a FULL glass kind of guy. We are sitting on the greatest advance in diabetes care in 100 years and have no plan. We are also sitting on something in the GLP1 market which showed great promise in Phase 1s and its sitting in the freezer. People have the GLP1 makers at full capacity so they can use it for weight loss.
I may have no vision or maybe I just don't like wasting time on dead-ends. I remember the last time Mike set up a big diversion at the share holders meeting and ran a skit with Damon Dash. What did that and Mr OneDrop do for MNKD, ZERO! I think we are seeing another diversion. Why are we dicking around with a product which if we get lucky we could make a few million when we have products which if properly marketed and sold could make billions?
BTW - what do you think of the label? Have you read it or did you just assume it was so bad??? The one big thing which the competition did extremely well after approval was they flooded the industry with all the nonsense about the black box and how dangerous afrezza was for the lungs. People talked of lungs exploding. I am still waiting to hear of one exploding lung case. They also told the doctors MNKD was going out of business and you will still see people on social media discuss how they asked their doctor for afrezza and the response was the company making it went out of business.
|
|
|
|
Post by Clement on Dec 15, 2022 14:56:52 GMT -5
Reps say, "You want cheap, we got cheap. You want the best, you have to pay for it."
|
|
|
|
Post by sayhey24 on Dec 15, 2022 15:05:47 GMT -5
IDK - $35 for afrezza is cheaper than the $35 RAA plus the V-Go. I have no idea what that costs on Medicare but they probably have to pay 20% of the cost on Plan B but I am guessing. Then again they still may want the Tresiba with the afrezza and that will be another $35. If they had been prescribed the afrezza much earlier they probably would not need the Tresiba.
|
|
|
|
Post by Thundersnow on Dec 15, 2022 16:45:53 GMT -5
Thundersnow - How much money did MNKD make or lose with V-Go last quarter? Was it a net loser and will it be a loser next quarter? How does a sales rep try and sell afrezza as the replacement to RAAs and then turn around and say I got a device here which will work with your RAA? What do the reps say - the RAAs are better than afrezza??? You can't have it both ways. They should be converting any doctor prescribing V-Go to afrezza. That $5M should be afrezza sales and they should be filling the dumpster with V-Gos. These should be easy targets. They know the doctors, they know V-Go and they should be able to easily explain why afrezza is far superior. I don't want a half full or half empty glass. I want a full glass. I am a FULL glass kind of guy. We are sitting on the greatest advance in diabetes care in 100 years and have no plan. We are also sitting on something in the GLP1 market which showed great promise in Phase 1s and its sitting in the freezer. People have the GLP1 makers at full capacity so they can use it for weight loss. I may have no vision or maybe I just don't like wasting time on dead-ends. I remember the last time Mike set up a big diversion at the share holders meeting and ran a skit with Damon Dash. What did that and Mr OneDrop do for MNKD, ZERO! I think we are seeing another diversion. Why are we dicking around with a product which if we get lucky we could make a few million when we have products which if properly marketed and sold could make billions? BTW - what do you think of the label? Have you read it or did you just assume it was so bad??? The one big thing which the competition did extremely well after approval was they flooded the industry with all the nonsense about the black box and how dangerous afrezza was for the lungs. People talked of lungs exploding. I am still waiting to hear of one exploding lung case. They also told the doctors MNKD was going out of business and you will still see people on social media discuss how they asked their doctor for afrezza and the response was the company making it went out of business. And YET YOU STILL OWN MNKD SHARES!!! What does that tell you??? Mike said VGO will be accretive next year so they will be profitable. VGO is giving MNKD GOVERNMENT MONEY.....Something that Afrezza is NOT DOING. VGO gives MNKD easy money and they are growing it. You are not going to convert the MEDICARE/AID diabetics over to Afrezza. Those are older patients set in their ways and will not try new therapies especially when you have Doctors not pushing it. Plain and simple therefore there is a strong and GROWING market for VGO. You keep bitchin' about the programs Mike initiated early in his tenure....Well when you have NO MONEY you have to grab the low hanging fruit like ONE DROP or DD and that Jamaican Chef. As you can see they are beyond that now and good things are in the works. Regarding GLP1 if my memory is correct Al Mann said the problem with an inhaled product it works too fast which is why they did not bring one to market. Maybe someone can back me up. The label is the label.....It's bad enough where by doctors are not convinced it's worth trying. Will that change?? Maybe, but they are going to need someone....FDA or GOD to tell them it's safe to use. Doctors rely on DATA, Studies and the ALMIGHTY ADA for guidance. Your BEEF is with the ADA not the Doctors, JDRF or any other company. I am done with this topic. But like I said....You still own MNKD shares for a reason. I TRUST Mike Castagna and believe he will make you money. |
|
|
|
Post by peppy on Dec 15, 2022 16:54:45 GMT -5
re-purpose V-GO outside diabetes? Do you mean like subQ Remodulin (treprostinil)? Or, Subcutaneous Immunoglobulin (SCIg)? I don't know anything you don't know (about MNKD plans). I'm just throwing out a couple of examples. I looked. No can do. Remodeling is given by an indwelling catheter. Not the same and not comparable, as it is given in Nanograms/kilo/min. ----------------------------INDICATIONS AND USAGE--------------------------- Remodulin is a prostacyclin mimetic indicated for: • Treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to diminish symptoms associated with exercise. Studies establishing effectiveness included patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (58%), PAH associated with congenital systemic-to-pulmonary shunts (23%), or PAH associated with connective tissue diseases (19%). (1.1) • Patients who require transition from epoprostenol, to reduce the rate of clinical deterioration. The risks and benefits of each drug should be carefully considered prior to transition. (1.2) ----------------------DOSAGE AND ADMINISTRATION----------------------- PAH WHO Group 1 in patients with NYHA Class II-IV symptoms: • Initial dose for patients new to prostacyclin infusion therapy: 1.25 ng/kg/min; increase based on clinical response (increments of 1.25 ng/kg/min per week for the first 4 weeks of treatment, later 2.5 ng/kg/min per week). Avoid abrupt cessation. (2.2, 2.4) • Mild to moderate hepatic insufficiency: Decrease initial dose to 0.625 ng/kg/min. Severe hepatic insufficiency: No studies performed. (2.5) Nontunneled lines are inserted directly into the desired vessel, and tunneled central venous catheters (CVCs) are placed at a more distant site and tunneled subcutaneously before entering the vessel. |
|
|
|
Post by sportsrancho on Dec 15, 2022 18:01:33 GMT -5
Most of the time, the doctors don’t have enough time with the patients to teach them and incorporate the right protocols on the dosing..
Sayhey has defended this company and Afrezza for over a decade! He’s had patience beyond compare.
I don’t blame the company for poor sales initially but the inability to develop a new and better strategy is what’s needed.
|
|
|
|
Post by sayhey24 on Dec 15, 2022 20:53:02 GMT -5
Thundersnow - How much money did MNKD make or lose with V-Go last quarter? Was it a net loser and will it be a loser next quarter? How does a sales rep try and sell afrezza as the replacement to RAAs and then turn around and say I got a device here which will work with your RAA? What do the reps say - the RAAs are better than afrezza??? You can't have it both ways. They should be converting any doctor prescribing V-Go to afrezza. That $5M should be afrezza sales and they should be filling the dumpster with V-Gos. These should be easy targets. They know the doctors, they know V-Go and they should be able to easily explain why afrezza is far superior. I don't want a half full or half empty glass. I want a full glass. I am a FULL glass kind of guy. We are sitting on the greatest advance in diabetes care in 100 years and have no plan. We are also sitting on something in the GLP1 market which showed great promise in Phase 1s and its sitting in the freezer. People have the GLP1 makers at full capacity so they can use it for weight loss. I may have no vision or maybe I just don't like wasting time on dead-ends. I remember the last time Mike set up a big diversion at the share holders meeting and ran a skit with Damon Dash. What did that and Mr OneDrop do for MNKD, ZERO! I think we are seeing another diversion. Why are we dicking around with a product which if we get lucky we could make a few million when we have products which if properly marketed and sold could make billions? BTW - what do you think of the label? Have you read it or did you just assume it was so bad??? The one big thing which the competition did extremely well after approval was they flooded the industry with all the nonsense about the black box and how dangerous afrezza was for the lungs. People talked of lungs exploding. I am still waiting to hear of one exploding lung case. They also told the doctors MNKD was going out of business and you will still see people on social media discuss how they asked their doctor for afrezza and the response was the company making it went out of business. And YET YOU STILL OWN MNKD SHARES!!! What does that tell you??? Mike said VGO will be accretive next year so they will be profitable. VGO is giving MNKD GOVERNMENT MONEY.....Something that Afrezza is NOT DOING. VGO gives MNKD easy money and they are growing it. You are not going to convert the MEDICARE/AID diabetics over to Afrezza. Those are older patients set in their ways and will not try new therapies especially when you have Doctors not pushing it. Plain and simple therefore there is a strong and GROWING market for VGO. You keep bitchin' about the programs Mike initiated early in his tenure....Well when you have NO MONEY you have to grab the low hanging fruit like ONE DROP or DD and that Jamaican Chef. As you can see they are beyond that now and good things are in the works. Regarding GLP1 if my memory is correct Al Mann said the problem with an inhaled product it works too fast which is why they did not bring one to market. Maybe someone can back me up. The label is the label.....It's bad enough where by doctors are not convinced it's worth trying. Will that change?? Maybe, but they are going to need someone....FDA or GOD to tell them it's safe to use. Doctors rely on DATA, Studies and the ALMIGHTY ADA for guidance. Your BEEF is with the ADA not the Doctors, JDRF or any other company. I am done with this topic. But like I said....You still own MNKD shares for a reason. I TRUST Mike Castagna and believe he will make you money. Yes - I still own shares. Lots and lots of shares. Call me crazy but I still believe Al Mann. What I have learned is afrezza is much better than I expected. The kids are going to start using afrezza after their trial and how crazy is that? If you look at the label that falls under the T1 section and that is were the label calls afrezza non-inferior. All the smart people back in 2014 said it was not even getting approved for T1s. I was not aware the Government was giving MNKD money for V-Go. Thats interesting - for what? Do we have a contract #? What is the Government doing with it and how much are we getting? If Mike can get a big government contract to repurpose V-Go I will be the first to say I was wrong and the purchase of V-Go was brilliant. I see Pfizer just got a $2B government deal for Paxlovid and thats on top of billons and billions for Covid vaccines. How much did MNKD get from the government for TS R&D??? If I remember correctly Mike told us we didn't want to get distracted with covid. Meanwhile we are sitting on TS. I really don't think Mike was grabbing any low hanging fruit at the share holders meeting. He was facing a group of very very angry shareholders and did a bait and switch with the diversion. He survived that meeting which I give him credit for. I even had fun taking pictures with Damon. Have we had a shareholders meeting in Danbury since? I had some hope in Jeff Dachis in fact I had a follow-up meeting and told my associate at the time this guy only wanted to sell his chrome meter. It was a nice meter but the time had come for CGMs and afrezza. In fact I just checked their website and he is still trying to sell the meter. How crazy is that? Regarding GLP1 did you read the patent info Mango posted? The patent addressed speed and the potential for 2 products. A long acting GLP1 like an Ozempic and a mealtime GLP1 which could be taken with afrezza. If my sources are correct it was put in the freezer because MNKD was short on money and was focused on getting afrezza approved. I am not sure Mike knew about it until this year. I suspect if asked Mike would just say it has no clinical benefit over existing GLP1s and he is too busy. This could be a great question for the quarterly call. Maybe I will ask and see what he says. Hey - if it can be as good as existing GLP1s and it could avoid the vomiting and injections - thats potential blockbuster in the diet space. Who is prescribing the GLP1s? Its the GPs and telemedicine. As Sports said they have no time to teach and worry about patients let alone injecting themselves. Aged got me reading all about GLP1s. I spent days. To be honest I still don't fully understand what they are fully doing but its not clear to me many do. They say they do a couple of things with the beta cells and increase insulin release yet T1s taking Ozempic are saying they use the same amount of afrezza at meals but less basal. I guess this might make sense if the GLP1 is slowing digestion but then why the same amount of afrezza? You would think the spike would be less. If what the T1s are seeing carry over to T2 this might be great with afrezza for the ones who nearing step 4. The more I think I understand the more questions I have. What I do know is we have no no pilots studies - none. My problem is not with the ADA. The ADA is paid by big pharma. The ADA and Big Pharma are a huge industry and the ADA is the front man for Big Pharma. Money talks and always will. Just in the T2 oral space its $40B. I think Dave Kendall found that out fast. My problem is we have a disruptive technology. In 6 years Mike has not figured out a plan to sell it into that market. His answer 3 years ago was he was shelving it for the T2s - the $40B market. Three years later its still shelved but they did a "Seeing and Believing Champaign" and spent $6M. Mike said it crashed and burned. Did they do any of this "Seeing is Believing" at ADA2022??? I don't think so. What did they do for $6M? How do you spend $6M in a couple of months??? I could get a megaphone and ladder on Amazon for less than $100 and sure could have gotten some attention at ADA2023. Here we are - No GLP1/afrezza study, no Moujarno study and no TS GLP1 study. Nothing. We spent $3B+ developing afrezza. Al spent $1B of his own money. When are we getting our money back plus dividends??? Instead Mike has spent time, resources and $20M+ on V-Go. Brilliant. He tells us V-Go its great. We can put it in the sales reps bag so when they can't figure out how to sell afrezza they can say that afrezza is no good anyway you really want the RAA and our mechanical dispenser. Is Mike telling us he can't figure out a plan for afrezza with the T2s and can't even find a partner to take another look at TS GLP1 and run it through Phase 2? I like Mike but if that is the case something has to change. We can't go on another 6 years like this. The pre-split price is less than a $1. Thats six years later - good grief! Is that what you call a bang up job? Todays closing price was $4.78 - that is 96cents and we are sitting on the greatest advance in diabetes care in over 100 years. He has done nothing with VDex, nothing with Ondou and nothing with anyone else that we have heard about. What did we hit at approval $55. Can we at least get half of that back? How about doing some deals and shaking some trees and target $25 for 12/2023. |
|
|
|
Post by Clement on Dec 15, 2022 21:24:08 GMT -5
re-purpose V-GO outside diabetes? Do you mean like subQ Remodulin (treprostinil)? Or, Subcutaneous Immunoglobulin (SCIg)? I don't know anything you don't know (about MNKD plans). I'm just throwing out a couple of examples. I looked. No can do. Remodeling is given by an indwelling catheter. Not the same and not comparable, as it is given in Nanograms/kilo/min. ----------------------------INDICATIONS AND USAGE--------------------------- Remodulin is a prostacyclin mimetic indicated for: • Treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to diminish symptoms associated with exercise. Studies establishing effectiveness included patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (58%), PAH associated with congenital systemic-to-pulmonary shunts (23%), or PAH associated with connective tissue diseases (19%). (1.1) • Patients who require transition from epoprostenol, to reduce the rate of clinical deterioration. The risks and benefits of each drug should be carefully considered prior to transition. (1.2) ----------------------DOSAGE AND ADMINISTRATION----------------------- PAH WHO Group 1 in patients with NYHA Class II-IV symptoms: • Initial dose for patients new to prostacyclin infusion therapy: 1.25 ng/kg/min; increase based on clinical response (increments of 1.25 ng/kg/min per week for the first 4 weeks of treatment, later 2.5 ng/kg/min per week). Avoid abrupt cessation. (2.2, 2.4) • Mild to moderate hepatic insufficiency: Decrease initial dose to 0.625 ng/kg/min. Severe hepatic insufficiency: No studies performed. (2.5) Nontunneled lines are inserted directly into the desired vessel, and tunneled central venous catheters (CVCs) are placed at a more distant site and tunneled subcutaneously before entering the vessel. There's a difference between IV and subQ. Remodulin can be administered IV or subQ. |
|
|
|
Post by cretin11 on Dec 15, 2022 23:44:12 GMT -5
I just wanna say thank you to sayhey. You have been in this for years, have been extremely patient, and have shared a lot of info with us. I wish you were employed by MNKD.
Minor quibble is that a couple of times you posted “maybe Mike is too busy” to do certain things. You don’t seem prone to sarcasm, but surely those have been sarcastic remarks, because “too busy” cannot accurately describe Mike.
Thank you again for posting honestly, even when some of your comments are not popular with everyone. This has been a very long road, many of us lost our taste for hopium years ago and prefer to keep things real now. The last couple of months have been nice but we are still nowhere near where we want and expect to be. It is pedal to the metal time.
|
|
|
|
Post by sayhey24 on Dec 16, 2022 8:51:28 GMT -5
I just wanna say thank you to sayhey. You have been in this for years, have been extremely patient, and have shared a lot of info with us. I wish you were employed by MNKD. Minor quibble is that a couple of times you posted “maybe Mike is too busy” to do certain things. You don’t seem prone to sarcasm, but surely those have been sarcastic remarks, because “too busy” cannot accurately describe Mike. Thank you again for posting honestly, even when some of your comments are not popular with everyone. This has been a very long road, many of us lost our taste for hopium years ago and prefer to keep things real now. The last couple of months have been nice but we are still nowhere near where we want and expect to be. It is pedal to the metal time. FYI - Mike is a busy guy. I am sure many have gotten emails or a phone call from Mike early in the morning when most are sleeping. Being busy and producing results are different. If I look at the results list its pretty thin. 1. Sale of the factory to raise cash 2. Hired a CFO to restructure debt 3. Took a phone call from Martine which saved the company from bankruptcy 4. Rescues V-Go from the dumpster and pays $15M+ for it. Either afrezza is as great as Al Mann said or its not. Mike says it is. VDex says it is. If we put PWDs on CGMs we can see the results. If its not then thats OK just tell the shareholders it was a big waste of $3B+ and we can move on. We can refocus to sell RAA's with our RAA dispenser. Mike said its going in the sales reps "bag" in January. It makes them sound like "Fuller Brush Man". Maybe they can sell soap and cleaning items too. The reality is three years ago afrezza was put on hold for the T2s. Thats a $40B market. Maybe Mike is working a deal. I sure hope he is. We have a study in India which looks the same as the Affinity-2 study - no CGMs, etc. I bet it will produce the same results - afrezza is superior to the orals. Back in the spring I started whining about how Mounjaro was going to kick our butt. I also thought it was interesting Dave Kendall left before the approval. Not a year later and the 2023 ADA SoC comes out and Boom - GIP is front and center. Have we done any pilot studies with or against Mounjaro? Then it got me thinking about the TS GLP1 MNKD had. Could it be used in the diet space? I am not sure Mike was even away they had done the phase 1s. Have you ever seen it on any of his slides??? GLP1s in diet are huge. I would think we could get some BP partner to take a look and run it through Phase 2 and see if Peter was correct. I hope he is working that deal but IDK. Maybe TS GLP1 and afrezza were both just big wastes of time and money and Al Mann was just an idiot. If that is what it is, OK. We just need to have Mike set us straight so we can focus on selling RAAs and our new RAA dispenser and whatever else he can put in the sales reps bag. |
|
|
|
Post by peppy on Dec 16, 2022 9:15:38 GMT -5
I looked. No can do. Remodeling is given by an indwelling catheter. Not the same and not comparable, as it is given in Nanograms/kilo/min. ----------------------------INDICATIONS AND USAGE--------------------------- Remodulin is a prostacyclin mimetic indicated for: • Treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to diminish symptoms associated with exercise. Studies establishing effectiveness included patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (58%), PAH associated with congenital systemic-to-pulmonary shunts (23%), or PAH associated with connective tissue diseases (19%). (1.1) • Patients who require transition from epoprostenol, to reduce the rate of clinical deterioration. The risks and benefits of each drug should be carefully considered prior to transition. (1.2) ----------------------DOSAGE AND ADMINISTRATION----------------------- PAH WHO Group 1 in patients with NYHA Class II-IV symptoms: • Initial dose for patients new to prostacyclin infusion therapy: 1.25 ng/kg/min; increase based on clinical response (increments of 1.25 ng/kg/min per week for the first 4 weeks of treatment, later 2.5 ng/kg/min per week). Avoid abrupt cessation. (2.2, 2.4) • Mild to moderate hepatic insufficiency: Decrease initial dose to 0.625 ng/kg/min. Severe hepatic insufficiency: No studies performed. (2.5) Nontunneled lines are inserted directly into the desired vessel, and tunneled central venous catheters (CVCs) are placed at a more distant site and tunneled subcutaneously before entering the vessel. There's a difference between IV and subQ. Remodulin can be administered IV or subQ.I missed that, were is that written? If it can be given subq, than, it /VGO could be potentially repurposed? |
|
|
|
Post by Clement on Dec 16, 2022 9:48:50 GMT -5
|
|
|
|
Post by peppy on Dec 16, 2022 9:58:47 GMT -5
Be still my heart, you sleuthed it out. sayhey24 stevil , can VGO be used for the Remodulin? mango 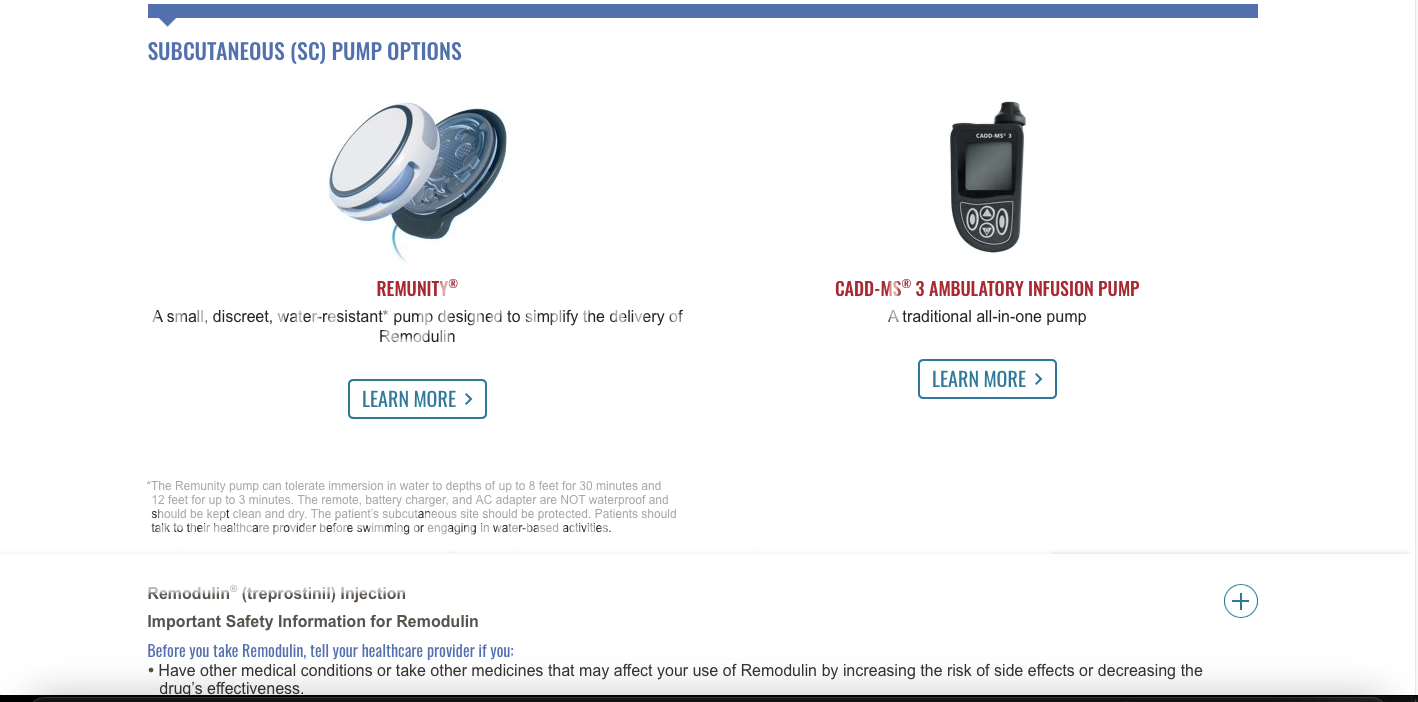 added, ----------------------DOSAGE AND ADMINISTRATION----------------------- PAH WHO Group 1 in patients with NYHA Class II-IV symptoms: • Initial dose for patients new to prostacyclin infusion therapy: 1.25 ng/kg/min; increase based on clinical response (increments of 1.25 ng/kg/min per week for the first 4 weeks of treatment, later 2.5 ng/kg/min per week). Avoid abrupt cessation. (2.2, 2.4) • Mild to moderate hepatic insufficiency: Decrease initial dose to 0.625 ng/kg/min. Severe hepatic insufficiency: No studies performed. (2.5) IV infusion of Remodulin delivered through an external pump has been associated with the risk of blood stream infections, arm swelling, tingling sensations, bruising, and pain. The most common side effects seen with either SC or IV Remodulin were headache, diarrhea, nausea, rash, jaw pain, widening of the blood vessels (vasodilatation), and swelling from fluid retention (edema). |
|
|
|
Post by uvula on Dec 16, 2022 11:12:55 GMT -5
Maybe mnkd bought vgo so that lqda couldn't buy it.
|
|




