|
|
Post by yossarian on Apr 28, 2016 1:26:04 GMT -5
|
|
|
|
Post by rockstarrick on Apr 28, 2016 3:15:32 GMT -5
It would be great to see sales in both of those countries by the end of the year, or early 2017.
I know Canada has been asking for it.
This would be a good thread to discuss the process and cost for distribution of Afrezza to these countries that accept FDA approval.
|
|
|
|
Post by mnkdfann on Apr 28, 2016 9:45:12 GMT -5
Nice to know Canada and Australia will accept FDA approval as their own for approval of drugs. He said some jurisdictions (like Canada and Australia) accept FDA approvals without the need to run new clinical trials. But, in fact, there are drugs approved by the FDA in the U.S. that are not approved by Health Canada. (And vice versa, for that matter.) So it would be more accurate to say that Health Canada might accept FDA approval as its own, but it does not have to. Furthermore: "the drug approval process in Canada is fragmented, as Ottawa is responsible for looking at the safety and efficacy of the drug, while the provinces look at the drug’s cost-effectiveness and makes the ultimate decision on whether it will be funded". So even if Health Canada approves the drug, the provinces still have to be lobbied and convinced by Mannkind to accept Afrezza. |
|
|
|
Post by peppy on Apr 28, 2016 9:58:01 GMT -5
Nice to know Canada and Australia will accept FDA approval as their own for approval of drugs. He said some jurisdictions (like Canada and Australia) accept FDA approvals without the need to run new clinical trials. But, in fact, there are drugs approved by the FDA in the U.S. that are not approved by Health Canada. (And vice versa, for that matter.) So it would be more accurate to say that Health Canada might accept FDA approval as its own, but it does not have to. Furthermore: "the drug approval process in Canada is fragmented, as Ottawa is responsible for looking at the safety and efficacy of the drug, while the provinces look at the drug’s cost-effectiveness and makes the ultimate decision on whether it will be funded". So even if Health Canada approves the drug, the provinces still have to be lobbied and convinced by Mannkind to accept Afrezza.
What do you think will need to be done with price for Afrezza for Health Canada to incorporate this insulin into their formularies? 35 million people live in Canada.
|
|
|
|
Post by nylefty on Apr 28, 2016 10:21:17 GMT -5
www.cbc.ca/news/health/prescription-drug-prices-1.3239317Patented drugs fall under the authority of [Canada's] Patented Medicine Prices Review Board (PMPRB), a federal agency which sets a price based on what the same drug costs in other countries. The price of generic or off-patent drugs is influenced by the individual provincial health-care systems.
Canada's maximum price for any given patented drug is the median of what the drug goes for in France, Italy, Sweden, Switzerland, Germany, the U.K and the U.S. The price stays locked, rising only with inflation.
|
|
|
|
Post by _neil on Apr 28, 2016 10:28:34 GMT -5
nylefty- That is good information. They do have an incentive then to not just lower the price on the ticket but do it through a carefully orchestrated discount program so they get maximum revenue from Health Canada. I don't know if PMPRB is that easy to fool but that could be a reason for MNKD not being upfront about saying they will lower the price. (I am feeling generous today.. normally I would accuse them of not having the foresight or the smarts to pull off anything to add revenue)
|
|
|
|
Post by kc on Apr 28, 2016 10:59:08 GMT -5
It would be great to see sales in both of those countries by the end of the year, or early 2017. I know Canada has been asking for it. This would be a good thread to discuss the process and cost for distribution of Afrezza to these countries that accept FDA approval. With Canada having a socialized medical program I am sure that MannKind would have to price it very competitively to get into their formulary. But they have plenty of room to do that at 100% of the revenue. They just need to get some very good successes behind them and picking up a country like Canada would be a big one. I hope they are focusing where the entry is easy. |
|
|
|
Post by kc on Apr 28, 2016 11:10:14 GMT -5
He said some jurisdictions (like Canada and Australia) accept FDA approvals without the need to run new clinical trials. But, in fact, there are drugs approved by the FDA in the U.S. that are not approved by Health Canada. (And vice versa, for that matter.) So it would be more accurate to say that Health Canada might accept FDA approval as its own, but it does not have to. Furthermore: "the drug approval process in Canada is fragmented, as Ottawa is responsible for looking at the safety and efficacy of the drug, while the provinces look at the drug’s cost-effectiveness and makes the ultimate decision on whether it will be funded". So even if Health Canada approves the drug, the provinces still have to be lobbied and convinced by Mannkind to accept Afrezza.
What do you think will need to be done with price for Afrezza for Health Canada to incorporate this insulin into their formularies? 35 million people live in Canada.
Price it cheaply for Health Canada. No reason not to price it right as we have the plant capacity to service it and it keeps production running. MannKind needs to have some really big successes to document the effectiveness of Afrezza in a big country. Canada would be the perfect place as they could really target the urban areas in a fast track manner. |
|
|
|
Post by peppy on Apr 28, 2016 11:18:54 GMT -5
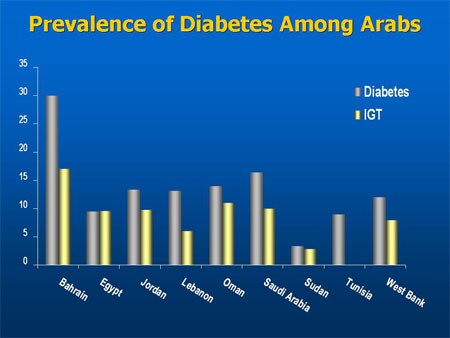
diabetes prevalence; China and/or India hits large populations. Ability to pay not known my me. this chart is obviously type 1 and type 2 (IGT shown on the second chart is glucose intolerance)

|
|
|
|
Post by matt on Apr 28, 2016 11:25:52 GMT -5
Price it cheaply for Health Canada. No reason not to price it right as we have the plant capacity to service it and it keeps production running. MannKind needs to have some really big successes to document the effectiveness of Afrezza in a big country. Canada would be the perfect place as they could really target the urban areas in a fast track manner. You do have to be a bit careful in pricing to gain entry to a single country. As noted above, Canada prices at or below the median price of a group of countries with decent pricing (UK and Italy are low). Japan caps reimbursement based on foreign average prices in US, UK, France, and Germany. Some others take a similar approach but include China and India. The point here is that a sweetheart deal for Canada can ripple though to pricing for other countries whether intended or not. You have to leave a few good markets where the company can charge an appropriate price because Afrezza costs more to manufacture. |
|
|
|
Post by sweedee79 on Apr 28, 2016 11:31:31 GMT -5
Nice to know Canada and Australia will accept FDA approval as their own for approval of drugs. He said some jurisdictions (like Canada and Australia) accept FDA approvals without the need to run new clinical trials. But, in fact, there are drugs approved by the FDA in the U.S. that are not approved by Health Canada. (And vice versa, for that matter.) So it would be more accurate to say that Health Canada might accept FDA approval as its own, but it does not have to. Furthermore: "the drug approval process in Canada is fragmented, as Ottawa is responsible for looking at the safety and efficacy of the drug, while the provinces look at the drug’s cost-effectiveness and makes the ultimate decision on whether it will be funded". So even if Health Canada approves the drug, the provinces still have to be lobbied and convinced by Mannkind to accept Afrezza. Would the label restrictions be as much of an issue in Canada and Australia as it is in the US? It would be nice if we could get a large group of people using Afrezza as it was intended to be used.. |
|
|
|
Post by agedhippie on Apr 28, 2016 13:41:11 GMT -5
What do you think will need to be done with price for Afrezza for Health Canada to incorporate this insulin into their formularies? 35 million people live in Canada.
Price it cheaply for Health Canada. No reason not to price it right as we have the plant capacity to service it and it keeps production running. MannKind needs to have some really big successes to document the effectiveness of Afrezza in a big country. Canada would be the perfect place as they could really target the urban areas in a fast track manner. Since Canadian pricing is apparently typically the median of the major European countries you are competing with Humalog where a box of 5 x 3ml KwikPen costs the NHS £29.46 or $47.13. Matching a box of 90 x 4u Afrezza would use 2 pens (6 x 3 x 30)/300 making $18.85. I have added an extra 2u per shot to account for priming. There are no rebate programs with the NHS, the price is the price. The European countries are all on-line since they share pricing information so it's hard for a pharma to vary the price. Can Mannkind hit $19 per box? |
|
|
|
Post by peppy on Apr 28, 2016 13:49:42 GMT -5
Price it cheaply for Health Canada. No reason not to price it right as we have the plant capacity to service it and it keeps production running. MannKind needs to have some really big successes to document the effectiveness of Afrezza in a big country. Canada would be the perfect place as they could really target the urban areas in a fast track manner. Since Canadian pricing is apparently typically the median of the major European countries you are competing with Humalog where a box of 5 x 3ml KwikPen costs the NHS £29.46 or $47.13. Matching a box of 90 x 4u Afrezza would use 2 pens (6 x 3 x 30)/300 making $18.85. I have added an extra 2u per shot to account for priming. There are no rebate programs with the NHS, the price is the price. The European countries are all on-line since they share pricing information so it's hard for a pharma to vary the price. Can Mannkind hit $19 per box? Aged you know a lot about this subject. You know MNKD can not hit $19 a box. A vial of apidra, I remember reading, with discount card the best the person could do is 100 dollars.
From a website: I live in Georgetown, Guyana. I have been getting my supplies of insulin from the Shopper's Drug Mart in Canada for the last 12 years.
I have been paying $35-$40 per vial of Apidra.
I am currently in vacation in Florida, and ran out of insulin, so I had to get a prescription to get one here.
To my surprise, the cost of one of the exact same vials here is $235.99.
How is that even possible.. does anyone know why it's so much over here? -----------------------------------------------------------------------------------------------------
How is it the pen is so much cheaper for fast acting?
MNKD, Afrezza could get by on $100 dollar 30 day 4 unit cartridges I think.
I recall reading the best this person could do in the USA for apidra with discount card was over $100 dollar, like $125 for a vial. A 30 day supply.
|
|
|
|
Post by mnkdfann on Apr 28, 2016 13:54:49 GMT -5
Price it cheaply for Health Canada. No reason not to price it right as we have the plant capacity to service it and it keeps production running. MannKind needs to have some really big successes to document the effectiveness of Afrezza in a big country. Canada would be the perfect place as they could really target the urban areas in a fast track manner. Since Canadian pricing is apparently typically the median of the major European countries you are competing with Humalog where a box of 5 x 3ml KwikPen costs the NHS £29.46 or $47.13. Matching a box of 90 x 4u Afrezza would use 2 pens (6 x 3 x 30)/300 making $18.85. I have added an extra 2u per shot to account for priming. There are no rebate programs with the NHS, the price is the price. The European countries are all on-line since they share pricing information so it's hard for a pharma to vary the price. The CBC article notes that "drug companies are no longer being transparent about prices. Companies now typically maintain a standard price for a drug across all territories, according to Morgan, then quietly arrange rebates or discounts with each country's regulator. The result? A regulator like the PMPRB will base a drug's maximum price on its listed price in France, Germany and elsewhere, but will have no idea how much money those countries might have been rebated in an undisclosed deal with the drug company." My reading of this is that the on-line info you mention would not reflect these undisclosed rebates. |
|
|
|
Post by agedhippie on Apr 28, 2016 14:18:10 GMT -5
Since Canadian pricing is apparently typically the median of the major European countries you are competing with Humalog where a box of 5 x 3ml KwikPen costs the NHS £29.46 or $47.13. Matching a box of 90 x 4u Afrezza would use 2 pens (6 x 3 x 30)/300 making $18.85. I have added an extra 2u per shot to account for priming. There are no rebate programs with the NHS, the price is the price. The European countries are all on-line since they share pricing information so it's hard for a pharma to vary the price. Can Mannkind hit $19 per box? Aged you know a lot about this subject. You know MNKD can not hit $19 a box. A vial of apidra, I remember reading, with discount card the best the person could do is 100 dollars.
From a website: I live in Georgetown, Guyana. I have been getting my supplies of insulin from the Shopper's Drug Mart in Canada for the last 12 years.
I have been paying $35-$40 per vial of Apidra.
I am currently in vacation in Florida, and ran out of insulin, so I had to get a prescription to get one here.
To my surprise, the cost of one of the exact same vials here is $235.99.
How is that even possible.. does anyone know why it's so much over here? -----------------------------------------------------------------------------------------------------
How is it the pen is so much cheaper for fast acting?
MNKD, Afrezza could get by on $100 dollar 30 day 4 unit cartridges I think.
I recall reading the best this person could do in the USA for apidra with discount card was over $100 dollar, like $125 for a vial. A 30 day supply.
I know Mannkind cannot hit $19 a box at the moment. It just seems that a lot of people are clinging to the idea that foreign markets, and Europe is often mentioned, is going to save them and it really isn't. The problem with most of the world is that there are national health systems so they can leverage market scale to reduce price. Tresiba just got withdrawn from Germany because the German health system refused to meet their price. To Germany this is not a problem because they have Lantus/Toujeo, Levemir, soon biosimilars all of who will meet their price. In the UK the NHS is now buying the new cancer drugs on a no cure no pay basis and the pharmas are going with it because they cannot afford to lose a market that size. The health system in the US is a trainwreck. We pay stupid prices because the largest buyer, the US government in the form of the VA or Medicare, is not allowed to negotiate. This allows the pharmas to jack up prices without penalty and the PBMs don't really care because for the most part they pass through the cost to the insurers and since you have to have insurance it lands on you in the end. Sorry, but this is a rant of mine.... |
|