|
|
Post by cjm18 on Sept 21, 2016 7:43:43 GMT -5
|
|
|
|
Post by mnholdem on Sept 21, 2016 7:48:38 GMT -5
|
|
|
|
Post by dh4mizzou on Sept 21, 2016 7:53:36 GMT -5
So is this a precursor to the FDA allowing "superior" status to Afrezza?
|
|
|
|
Post by therealisaching on Sept 21, 2016 8:20:25 GMT -5
Really strange that this study looks like it was done by Sanofi. Was this in their pocket the entire time? |
|
|
|
Post by me on Sept 21, 2016 8:25:32 GMT -5
I only perused the article, but since the study appeared to be done by (primarily) SNY scientists, I wonder when the research was actually completed (as opposed to the publish date)?
|
|
|
|
Post by gonetotown on Sept 21, 2016 8:35:58 GMT -5
Looks to me it's all computer simulation.
|
|
|
|
Post by straightly on Sept 21, 2016 9:25:43 GMT -5
Looks to me it's all computer simulation. By adapting the dosing regimen and titration rule, however, an additional reduction of 0.5% in HbA1c could be achieved, suggesting that the optimal dosing of TI can be more efficacious than an SC-injected prandial insulin analog, while keeping a reduced risk of hypoglycemia. Clinical studies are currently planned to validate the results from these in-silico meal test simulations in T1D. |
|
|
|
Post by peppy on Sept 21, 2016 9:36:14 GMT -5
|
|
|
|
Post by goyocafe on Sept 21, 2016 9:40:47 GMT -5
Looks to me it's all computer simulation. By adapting the dosing regimen and titration rule, however, an additional reduction of 0.5% in HbA1c could be achieved, suggesting that the optimal dosing of TI can be more efficacious than an SC-injected prandial insulin analog, while keeping a reduced risk of hypoglycemia. Clinical studies are currently planned to validate the results from these in-silico meal test simulations in T1D. Is that an additional reduction of HbA1c based on the poorly designed trials that were used for approval or based on dosing that is more in line with the known characteristics and timing of Afrezza? What some users are getting over their previous RAA is much greater than .5% reductions, so I'm guessing this dosing question is in addition to timing. |
|
|
|
Post by matt on Sept 21, 2016 11:28:22 GMT -5
Looks to me it's all computer simulation. Exactly right, " in silico" is Latin for "in silicon", hence the reference to virtual patients. Computer models are helpful to stimulate discussion in the academic community and to plan research studies, but FDA is going to want to see in vivo data from real patients before accepting a label change. The model presumes that all the human factors and the pharmacodynamics of each substance are fully known, but there is still a lot we do not know with any degree of certainty. |
|
|
|
Post by mnholdem on Sept 21, 2016 11:47:26 GMT -5
Looks to me it's all computer simulation. Exactly right, " in silico" is Latin for "in silicon", hence the reference to virtual patients. Computer models are helpful to stimulate discussion in the academic community and to plan research studies, but FDA is going to want to see in vivo data from real patients before accepting a label change. The model presumes that all the human factors and the pharmacodynamics of each substance are fully known, but there is still a lot we do not know with any degree of certainty. The authors' opinions appear to state what Matt posted (above). In-silico testing may be useful as a replacement for preclinical trials, typically performed in animals. The simulation would speed up the development of a new pipeline drug, but have little direct benefit in changing the label. However, titration methods typically are not included on the label and are left to the physicians to determine, based on the patients' needs. The authors specifically state that their in-silico testing is intended for establishing better titration rules.
Excerpt:
A clinical study to identify the optimal dosing regimen and the optimal titration rule would be prohibitively expensive because countless combinations would need to be tested. Thus, we have applied a cost-effective alternative and performed in-silico clinical trials using a validated T1D simulator that translates the known PK profile of TI (and insulin lispro as comparator) into the expected postprandial
glucose response following a meal tolerance test. In-silico testing with the T1D simulator allowed for exploration of different dosing regimens and different titration rules to guide virtual patients to their individual TI dose. It also allowed the performance of TI to be benchmarked against standard SC insulin analogs.
The simulations suggest that postmeal dosing and split dosing of TI can provide a flatter postprandial glucose profile with lower fluctuation amplitudes than at-meal dosing.
You can see from Fig. 4 in the report, many of the 100 patients simulated in the in-silico study show unacceptably high & low postmeal glucose levels when treated with insulin lispro (Graphs A and B). The simulator demonstrates that much better postmeal glucose control is achieved for a patient using either at-meal or post-meal dosing of Technosphere insulin (Graph C). 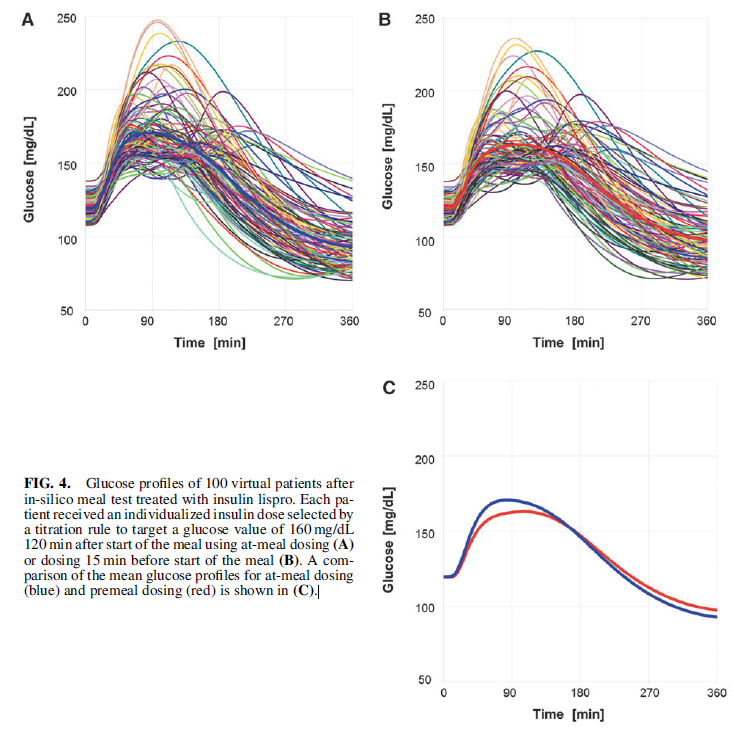
|
|
|
|
Post by derek2 on Sept 21, 2016 14:36:03 GMT -5
|
|
|
|
Post by compound26 on Sept 21, 2016 14:45:49 GMT -5
Yes, this was discussed before. See below. And I believe Matt/Mike has referred to this article in their recent presentations.
|
|
|
|
Post by peppy on Sept 21, 2016 14:46:48 GMT -5
yes he did. You found it Derek! wow, you found it.
|
|
|
|
Post by dreamboatcruise on Sept 21, 2016 14:52:16 GMT -5
Looks to me it's all computer simulation. Exactly right, " in silico" is Latin for "in silicon", hence the reference to virtual patients. Computer models are helpful to stimulate discussion in the academic community and to plan research studies, but FDA is going to want to see in vivo data from real patients before accepting a label change. The model presumes that all the human factors and the pharmacodynamics of each substance are fully known, but there is still a lot we do not know with any degree of certainty. Though if I'm not mistaken this particular simulation package has some sort of FDA approval. Thus it appears the FDA may be moving towards allowing simulations to play some role in the regulatory process. Obviously, it seems far fetched they replace clinical trials for approval, but perhaps they might be used for change in dosing guidelines? Though if there is zero precedence to date, it's a sure bet there would be resistance and an ongoing debate within the FDA. That sort of change doesn't occur without a conservative view fighting against it. As physiological simulation advances I think the benefit will be significant. Even if not used directly for regulatory action it could help design the clinical studies. At a minimum that is what MNKD is doing with this. |
|